Thymopentin inhalation powder aerosol and preparation method thereof
A technology for inhaling powder and thymus, which is applied in the field of thymopentin inhalation powder and its preparation, can solve the problems of thymopentin not easy to degrade, low bioavailability, large energy consumption, etc., to achieve rich blood flow, biological High utilization and pain relief effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1: the preparation of thymopentin inhalation powder spray
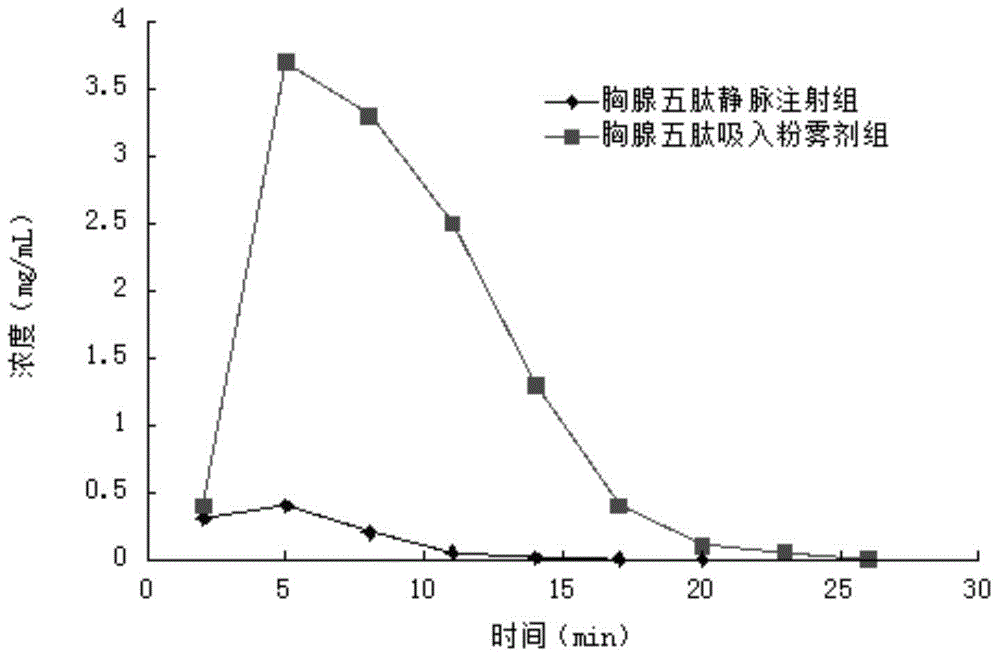
[0045] Grind 1g of Thymopentin finely, and use an ultrafine pulverizer to perform ultrafine pulverization to obtain an ultrafine dry powder with a particle diameter of 1 μm; grind 21.28g of mannitol, 106.42g of leucine, and 1.3g of poloxamer 188 at a high speed Pulverizer, high-speed grinding and pulverization, to obtain micropowder with a particle size of 60 μm ~ 80 μm; mix thymopentin ultrafine dry powder and auxiliary materials according to the method of equal increase, fill No. 5 capsules, each filling 130mg, and make 1000 capsules, namely The Thymopentin inhalation spray that gets the content of every dose of principal agent is 1mg.
Embodiment 2
[0046] Embodiment 2: the preparation of thymopentin inhalation powder spray
[0047] Grind 1g of Thymopentin finely, and use an ultrafine pulverizer to perform ultrafine pulverization to obtain an ultrafine dry powder with a particle size of 5 μm; grind 42.5g of mannitol, 85.2g of leucine, and 1.3g of poloxamer 188 at a high speed Pulverizer, high-speed grinding and crushing to obtain a particle size of 60 μm micropowder; Thymopentin ultrafine dry powder and auxiliary materials are mixed according to the method of equal increase, and filled with No. 5 capsules, each filled with 130mg, made into 1000 capsules, that is Thymopentin inhalation spray with a content of 1 mg main drug.
Embodiment 3
[0048] Embodiment 3: the preparation of thymopentin inhalation powder spray
[0049] Grind 0.5g of thymopentin finely, and ultrafinely pulverize with an ultrafine pulverizer to obtain an ultrafine dry powder with a particle diameter of 2 μm; mix 85.4g of mannitol, 42.7g of leucine, and 1.4g of poloxamer 188 with a high-speed Grinding and pulverizing with a high-speed grinding machine to obtain a micropowder with a particle size of 80 μm; mix the thymopentin ultrafine dry powder and auxiliary materials according to the method of equal increase, fill No. 5 capsules, each filling 130mg, and make 1000 capsules. The content of each main drug is 0.5 mg thymopentin inhalation spray.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com