Pharmaceutical composition for treating wound infection after pediatric fracture
A composition and medicine technology, applied in the field of medicine, can solve the problems that antibiotics are easy to produce drug resistance, high cost, great pain for patients, etc., and achieve the effects of promoting wound healing, safe and reliable effect, and infection control.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0014] Embodiment 1 Preparation of the pharmaceutical composition of the present invention
[0015] Taking ointment as an example, the preparation process steps of the pharmaceutical composition of the present invention are as follows:
[0016] (1) Weigh 80 grams of frankincense, 50 grams of centella asiatica, 150 grams of calabash vine, 120 grams of Eucommia ulmoides, 80 grams of licorice, 50 grams of argyi, 100 grams of sardine grass, 120 grams of burnet, and 80 grams of rose leaves , 150 grams of honeysuckle, 50 grams of quince, 100 grams of Panax notoginseng, 100 grams of betel, 150 grams of agave, 100 grams of coriander root, 50 grams of sheep ear garlic, 120 grams of safflower, 100 grams of silkworm, 150 grams of turban 100 grams of strong gluten grass, 100 grams of Buddha belly flower, 80 grams of dried blood;
[0017] (2) Crumble frankincense and dried blood into fine powder, pass through a 120-mesh sieve, and set aside;
experiment example
[0021] Experimental example Effect of the pharmaceutical composition of the present invention
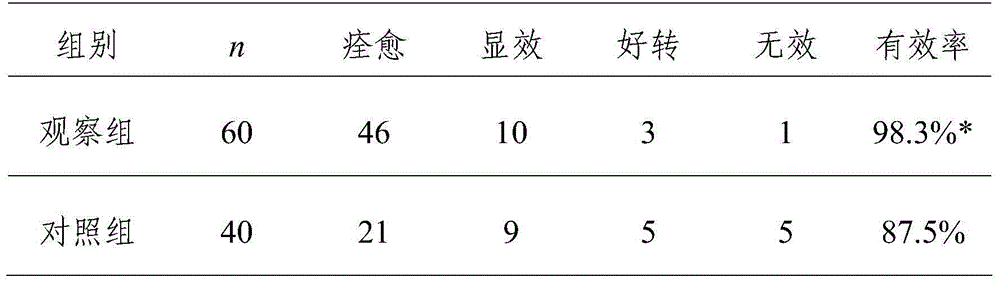
[0022] 1. General information In this group, 100 children with infected wounds after fracture surgery were divided into an observation group of 60 cases and a control group of 40 cases. The children in the observation group were between 3 and 8 years old, with an average of 6.5 years old; the infected wound area ranged from 0.4cm×2.2cm to 3.0cm×8.5cm, and 10 cases had skin and soft tissue defects with purulent secretions; no bone, Tendons and nerves are exposed. The age of the control group was between 2.5 and 9 years old, with an average of 6.2 years old; the infected wound area ranged from 0.3cm×2.0cm to 3.7cm×9.5cm, including 9 cases of skin and soft tissue defects with purulent secretions; no bone, tendon, Nerve exposure. All children suffered from infection after fracture operation. There was no significant difference in the general data of the two groups of patients (P>0.05...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com