Oral hexavalent reassorted rotavirus live vaccine
A rotavirus and live vaccine technology, applied in antiviral agents, viral antigen components, digestive system, etc., can solve the problems of small coverage and serotype coverage, and achieve the effect of facilitating operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1. Preparation of each serotype virus stock solution
[0046] Cell passage: The culture substrate of each serotype virus is Vero cells. First, resuscitate the working seeds of Vero cells. After culturing at 37°C for 3-5 days, carry out passage and expansion culture according to a certain ratio. The culture containers are T25, T75, and T175 cells respectively. Culture flasks, 10-layer cell factories, and 40-layer cell factories were inoculated with G1, G2, G3, G4, G8, and G9 serotype rotavirus after culturing 3-5 generations in the 40-layer cell factory.
[0047] Virus culture: After the cell confluence in the cell factory reaches more than 90%, the virus inoculation operation can be carried out. Before virus inoculation, select a cell factory for cell counting. According to the counting results, calculate according to MOI (multiplicity of virus infection) 0.005-0.2 to obtain the required amount of virus, and activate the virus with trypsin. The activation co...
Embodiment 2
[0050] Embodiment 2. Development of vaccine protective agent
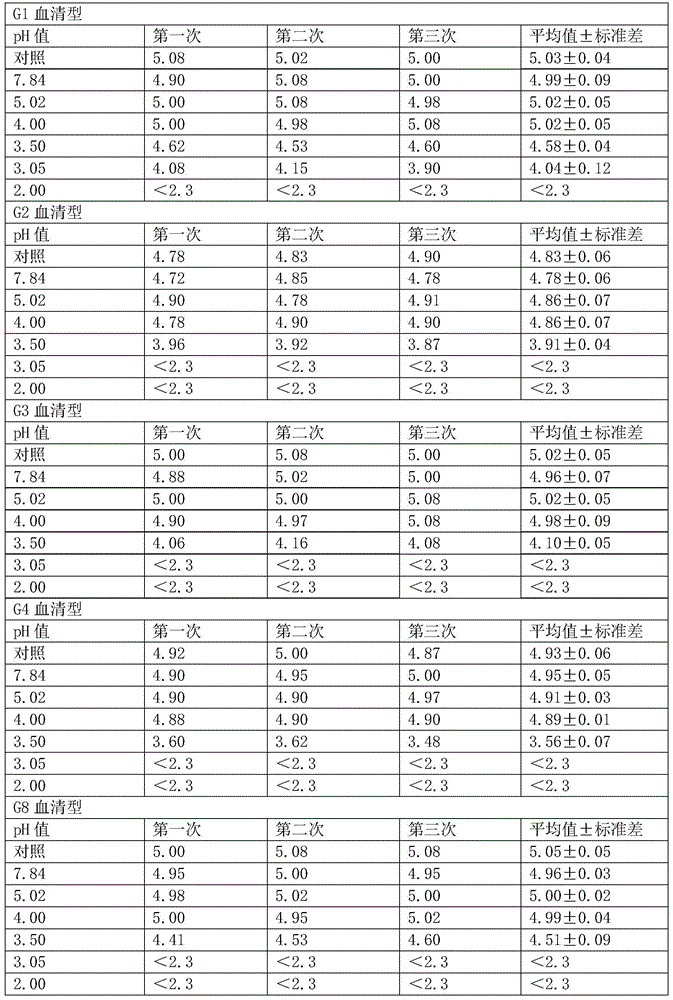
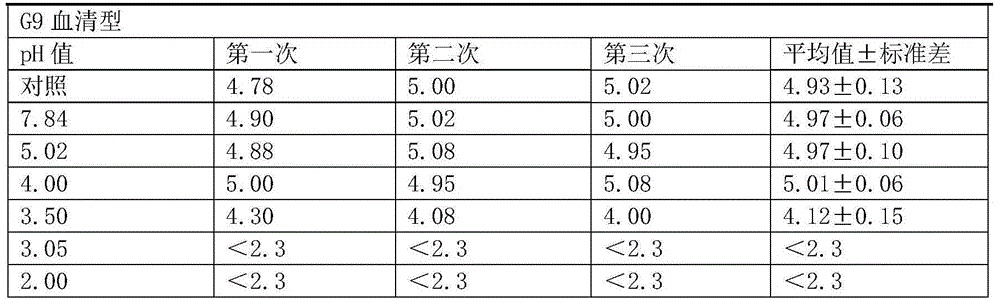
[0051] (1) Determination of virus resistance to acid
[0052] The minimum pH value that the hexavalent rotavirus vaccine can tolerate is determined by experiment.
[0053] Mix the hexavalent serotype virus mixed vaccine (the stock solution of the hexavalent vaccine is mixed in equal volumes) and 1% sodium citrate solution in equal volumes.
[0054] (2) Prepare 7 disposable sterile 50ml centrifuge tubes, 6 of which were added with 2ml of hexavalent seedlings mixed in step (1), and the other 1 was added with 1ml of hexavalent mixed seedlings and 1ml of DMEM as a control.
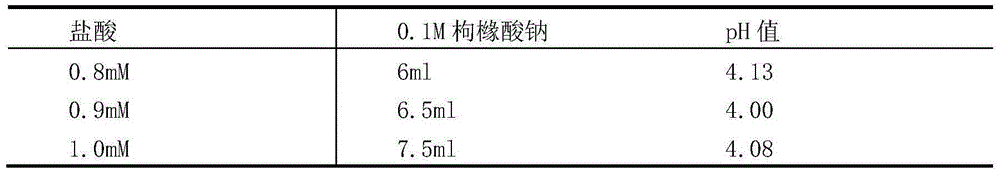
[0055] (3) To adjust the pH, add 0.1M hydrochloric acid 0ml, 0.4ml, 0.6ml, 0.7ml, 0.8ml, 1.2ml to 6 centrifuge tubes respectively, and the corresponding pH values are 7.84, 5.02, 4.00, 3.50, 3.05, 2.00 .
[0056] (4) Place 7 centrifuge tubes in a 37°C water bath and incubate for 2 hours.
[0057] (5) After the incubation time is over, immedia...
Embodiment 3
[0086] Example 3. Screening of different formulations
[0087] See Table 4 for the composition contents of 5 kinds of vaccine protective agent formulations
[0088] Table 4 Contents of each component of different protective agents
[0089]
[0090] Each formula was mixed with the most temperature-sensitive serotype G9 virus, and an accelerated stability test was carried out. The results are shown in Table 5
[0091] Table 5 Virus stability test of different protection formulations (all data are the mean value of three test results, unit: lgFFU / ml)
[0092]
[0093] It can be seen that the protection of the above five formulas to G9 all meets the requirements, among which F5 is stored at 37°C for 7 days, the drop in G9 titer is the lowest, and the protection to G9 is the best, so F5 is the best for rotavirus vaccine Preservative formula.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com