Artificial chylomicron containing medicine carrying compound as well as preparation method and application thereof
A composition, artificial technology, applied in the direction of drug combination, pharmaceutical formula, medical preparations of inactive ingredients, etc., can solve the problems of lack of stability and targeting, poor stability, short retention time, etc., to increase passive The effect of targeting function and ensuring stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Prescription of artificial chylodrug-loaded composition:
[0066]
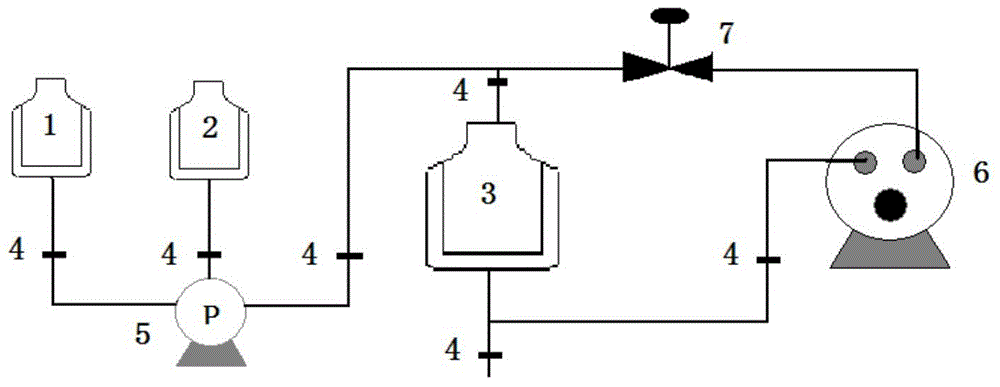
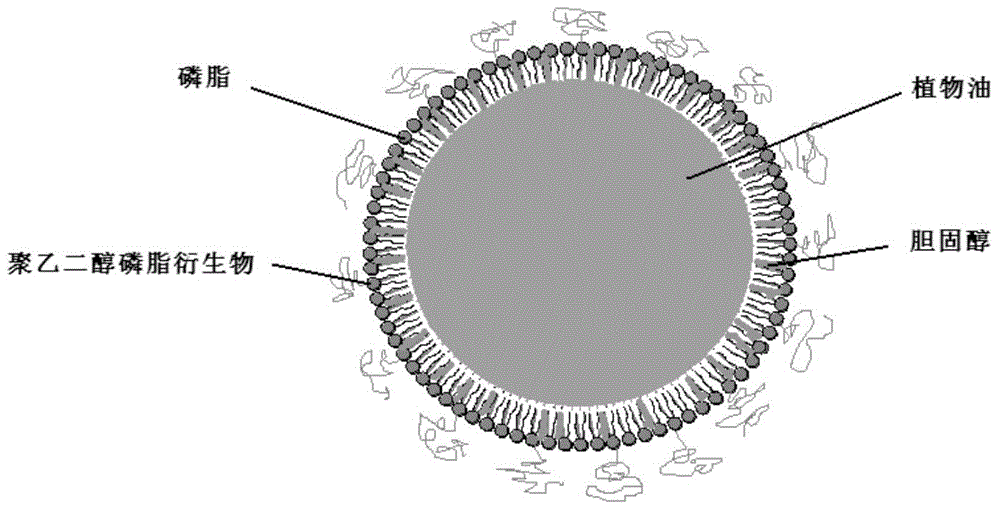
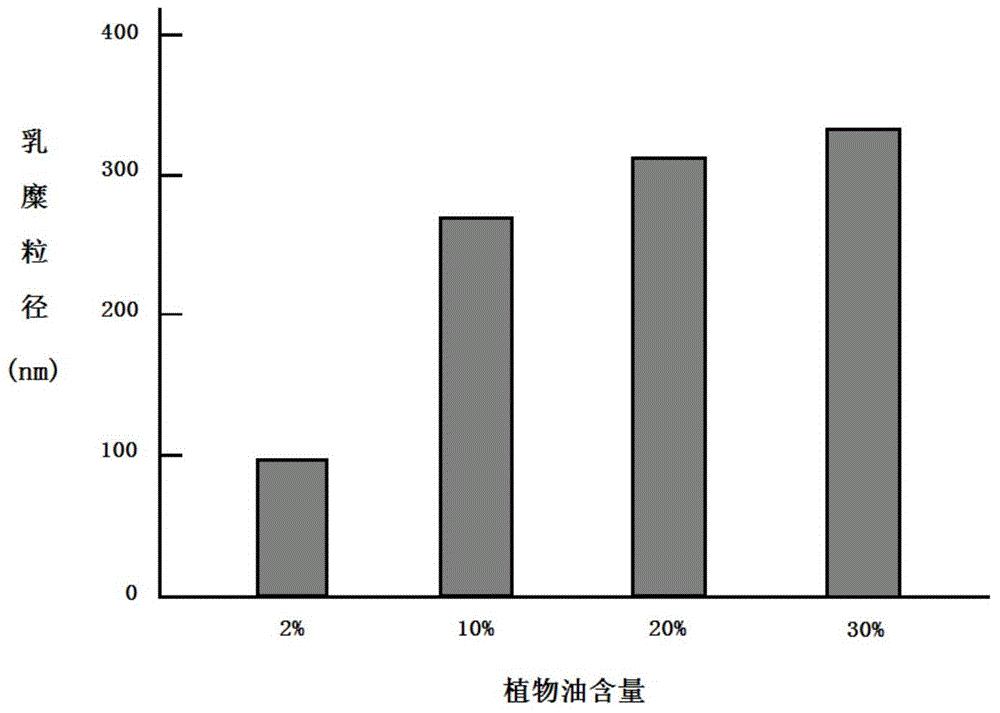
[0067] The preparation method is as follows: take 10.0g soybean oil, add 9.5g lecithin, 1.0g cholesterol, 0.5g sodium oleate, 2.5g polyethylene glycol-phospholipid (PEG2000-DSPE), and heat to 75 ℃, stir for about 20 minutes to fully dissolve the various compounds added, then add 5ml vitamin E, dissolve and mix well. Another 800ml of water for injection was taken, and 22g of glycerol was added. Under the protection of nitrogen, the oil solution was added into the aqueous glycerin solution under the condition of shearing and stirring to prepare colostrum, and the total amount was adjusted to 1000ml. The high-pressure homogenizer is used for homogenization 5-15 times, the homogenization pressure is about 100MPa, the pH is adjusted to 6.0-8.0, filtered, sub-packaged, nitrogen gas is introduced, and sealed. Sterilize at 115°C for 30 minutes (or at 121°C for 15 minutes). After passing the light inspectio...
Embodiment 2
[0069] Prescription of artificial chylodrug-loaded composition:
[0070]
[0071] Its preparation method is: take olive oil 100.0g, add 9.5g lecithin, 1.0g cholesterol, 0.5g sodium oleate, 2.5g polyethylene glycol-phospholipid (PEG2000-DSPE), under the condition of nitrogen protection, heat to At 75°C, stir for about 20 minutes to fully dissolve the various compounds added, then add 5ml vitamin E, dissolve and mix well. Another 800ml of water for injection was taken, and 22g of glycerol was added. Under the condition of nitrogen protection, the oil solution was added into the glycerin aqueous solution under the condition of shearing and stirring to prepare colostrum, and the total amount was adjusted to 1000ml. The high-pressure homogenizer homogenizes for 5-8 times, the homogenization pressure is about 100MPa, adjusts the pH to 7.0-8.0, filters, subpackages, injects nitrogen, and seals. Rotate sterilization at 115°C for 30 minutes. After passing the light inspection, det...
Embodiment 3
[0073] Prescription of artificial chylodrug-loaded composition:
[0074]
[0075] Its preparation method is: take fish oil 100.0g, add 9.5g lecithin, 1.0g cholesterol, 0.5g sodium oleate, 2.5g polyethylene glycol-phospholipid (PEG2000-DSPE), under the condition of nitrogen protection, heat to 75 ℃, stir for about 20 minutes to fully dissolve the various compounds added, then add 5ml vitamin E, dissolve and mix well. Another 800ml of water for injection was taken, and 22g of glycerol was added. Under the condition of nitrogen protection, the oil solution was added into the glycerin aqueous solution under the condition of shearing and stirring to prepare colostrum, and the total amount was adjusted to 1000ml. The high-pressure homogenizer homogenizes for 5-8 times, the homogenization pressure is about 100MPa, adjusts the pH to 7.2-8.5, filters, packs, injects nitrogen, and seals. Sterilize at 115°C for 30 minutes. After passing the light inspection, test the fish oil conten...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com