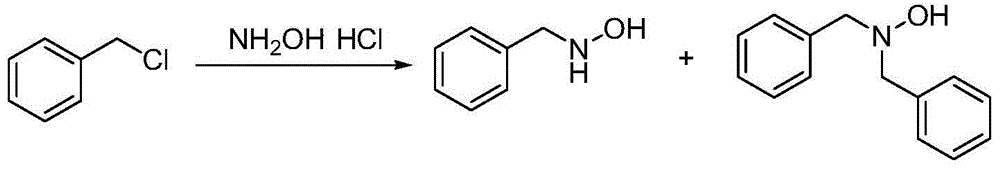
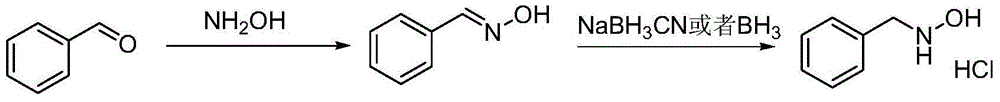
Method for synthesizing N-benzylhydroxylamine hydrochloride
A technology of benzyl hydroxylamine hydrochloride and benzyl nitrone, which is applied in the field of industrially synthesized preparation of N-benzyl hydroxylamine hydrochloride, can solve the problems of high price and low yield, and achieve simple operation and high reaction efficiency. The effect of high efficiency and product purity and stable conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 100 g of dibenzylamine, 500 mL of methanol and 3.0 g of sodium tungstate dihydrate into a 1.0 L four-necked reaction flask. While stirring, cool down to 0°C with an ice-salt bath. Slowly add 115mL of hydrogen peroxide dropwise into the reaction flask, and control the temperature <5°C during the dropwise addition. After the addition was complete, the mixture was incubated in an ice-water bath for 2 hours. The temperature was raised naturally, and the reaction was stirred overnight at room temperature. The reaction solution was slowly added to ice water. After suction filtration, the filter cake was dried at 45°C to obtain 91.4 g of C-phenyl-N-benzylnitrone, with a yield of 85.3%.
[0027] ESI: m / z=212[M+H]; 1 H NMR (400MHz, DMSO) δ8.28–8.19 (m, 2H), 8.10 (s, 1H), 7.50 (dd, J=7.7, 1.5Hz, 2H), 7.46–7.32 (m, 6H), 5.07 ( s,2H).
Embodiment 2
[0029] Add 91.4 g of C-phenyl-N-benzylnitrone and 455 mL of MTBE to a 2.0 L one-necked bottle. A solution of 29.8 g of hydroxylamine hydrochloride in methanol (320 mL) was added dropwise with stirring. After the addition, the reaction was stirred for 5h. The reaction solution was concentrated with a rotary evaporator. Add 455 mL of MTBE to the concentrated solid, stir and filter to obtain a white solid.
[0030] The white solid was dissolved in 66 mL of methanol under heating, and 330 mL of MTBE was added dropwise thereto. After cooling down to room temperature, a large amount of white solid was precipitated, which was filtered and dried to obtain 50 g of N-benzylhydroxylamine with a yield of 72.4%.
[0031] ESI: m / z=124[M+H]; 1 H NMR (400MHz, DMSO) δ11.77(br,2H), 10.98(br,1H), 7.60–7.46(m,2H), 7.46–7.35(m,3H), 4.30(s,2H).
Embodiment 3
[0033] Put 4.0kg of dibenzylamine, 20kg of methanol, and 120g of sodium tungstate dihydrate into the 50L reaction kettle in sequence. Start stirring, and cool down the temperature of the materials in the reactor to 0°C. Slowly add 4.6L of 30% hydrogen peroxide dropwise into the reactor, and control the temperature <5°C during the dropwise addition. After the addition was complete, the mixture was incubated in an ice-water bath for 2 hours. Take the reaction solution and detect the content of dibenzylamine therein by HPLC, when the content of dibenzylamine is less than 5%. The temperature was raised naturally, and stirred overnight at room temperature. The reaction solution was slowly added to 70 L of ice water. Stirring was continued for 1 h. After centrifugation, the filter cake was washed with 2L of water each time until the centrifuged liquid was detected to be free of peroxide with starch potassium iodide test paper. The filter cake was dried at 45°C to obtain 3.97kg ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com