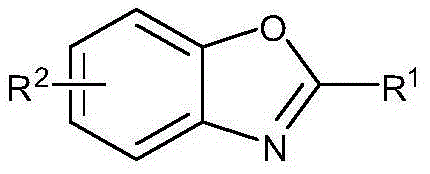
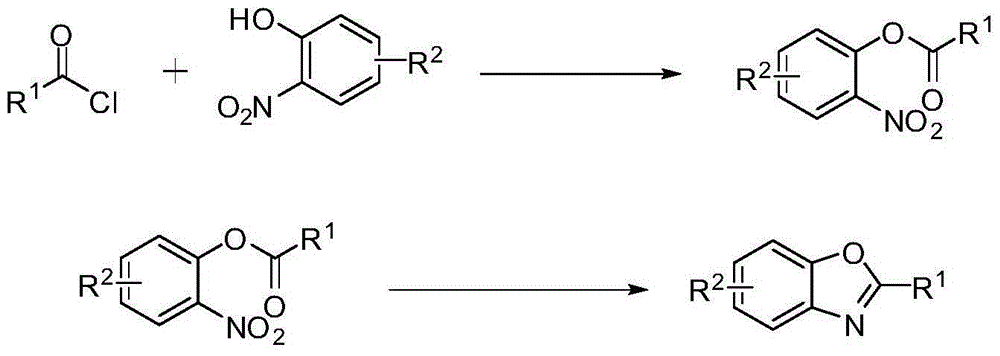
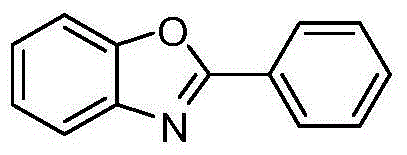
Preparing method of 2-substituted benzoxazole compounds
A compound and oxazole technology, applied in the field of preparation of 2-substituted benzoxazole compounds, can solve the problem that reactants are unstable, are not suitable for large-scale safe production of 2-substituted benzoxazole compounds, and are unfavorable. industrialization, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: the preparation of 2-phenylbenzoxazole
[0023]
[0024] (1) Synthesis of the substrate: Take 3.6 mmol o-nitrophenol into a flask, add 1 mL pyridine, mix well to obtain a dark brown liquid. Add 3.9 mmol of benzoyl chloride to obtain a light yellow emulsion, and stir magnetically for 4 h at room temperature. The product was extracted (water was added to quench the reaction, and saturated sodium chloride was added to prevent emulsification), and the organic phase was obtained by standing at room temperature, which was dried at room temperature for 1 hour. The obtained dried product was separated by column chromatography, and the eluted product was spin-dried using a rotary evaporator to obtain the reaction raw material.
[0025] (2) Synthesis of 2-phenylbenzoxazole: get 1.0 mmol of substrate and put it into a flask, add 4.0 mmol of ammonium formate, 20 mL of glacial acetic acid, and finally add 10% catalyst 5% palladium carbon of raw material content. R...
Embodiment 2
[0026] Embodiment 2: the preparation of 5-methyl-2-benzoxazole
[0027]
[0028] (1) Synthesis of the reaction substrate: 3.6 mmol of 4-methyl-2-nitrophenol was put into a flask, 1 mL of pyridine was added, and a dark brown liquid was obtained by uniform mixing. Add 3.9 mmol of benzoyl chloride to obtain a light yellow emulsion, and stir it magnetically for 4 hours at room temperature. The product was extracted (water was added to quench the reaction, and saturated sodium chloride was added to prevent emulsification), and the organic phase was obtained by standing at room temperature, which was dried at room temperature for 1 hour. The obtained dried product was separated by column chromatography, and the eluted product was spin-dried using a rotary evaporator to obtain the reaction raw material.
[0029] (2) Preparation of target compound: put 1.0 mmol of substrate into a flask, add 4.0 mmol of ammonium formate, 20 mL of glacial acetic acid, and finally add catalyst palla...
Embodiment 3
[0030] Example 3: 5-tert-butyl-2-benzoxazole
[0031]
[0032] (1) Synthesis of reaction substrate: Take 3.6 mmol of 4-tert-butyl-2-nitrophenol into a flask, add 1 mL of pyridine, and mix uniformly to obtain a dark brown liquid. Add 3.9 mmol of benzoyl chloride to obtain a light yellow emulsion, and stir it magnetically for 4 hours at room temperature. The product was extracted (water was added to quench the reaction, and saturated sodium chloride was added to prevent emulsification), and the organic phase was obtained by standing at room temperature, which was dried at room temperature for 1 hour. The obtained dried product was separated by column chromatography, and the eluted product was spin-dried using a rotary evaporator to obtain the reaction raw material.
[0033] (2) Preparation of target compound: put 1.0 mmol of substrate into a flask, add 4.0 mmol of ammonium formate, 20 mL of glacial acetic acid, and finally add catalyst palladium carbon with 10% of raw materi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com