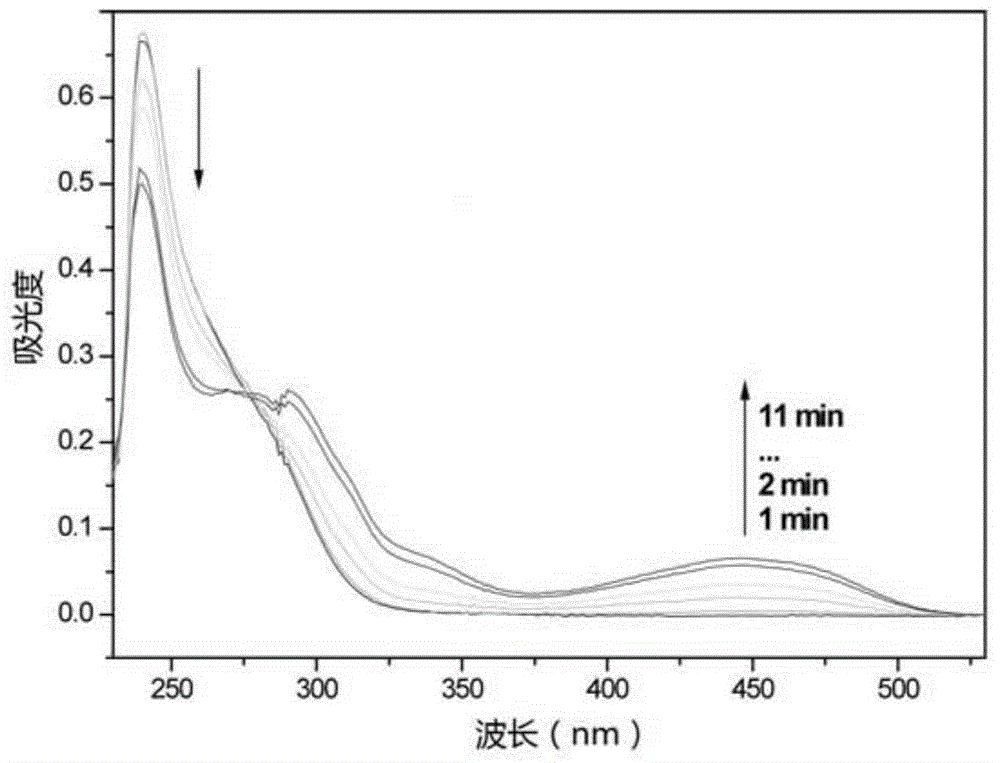
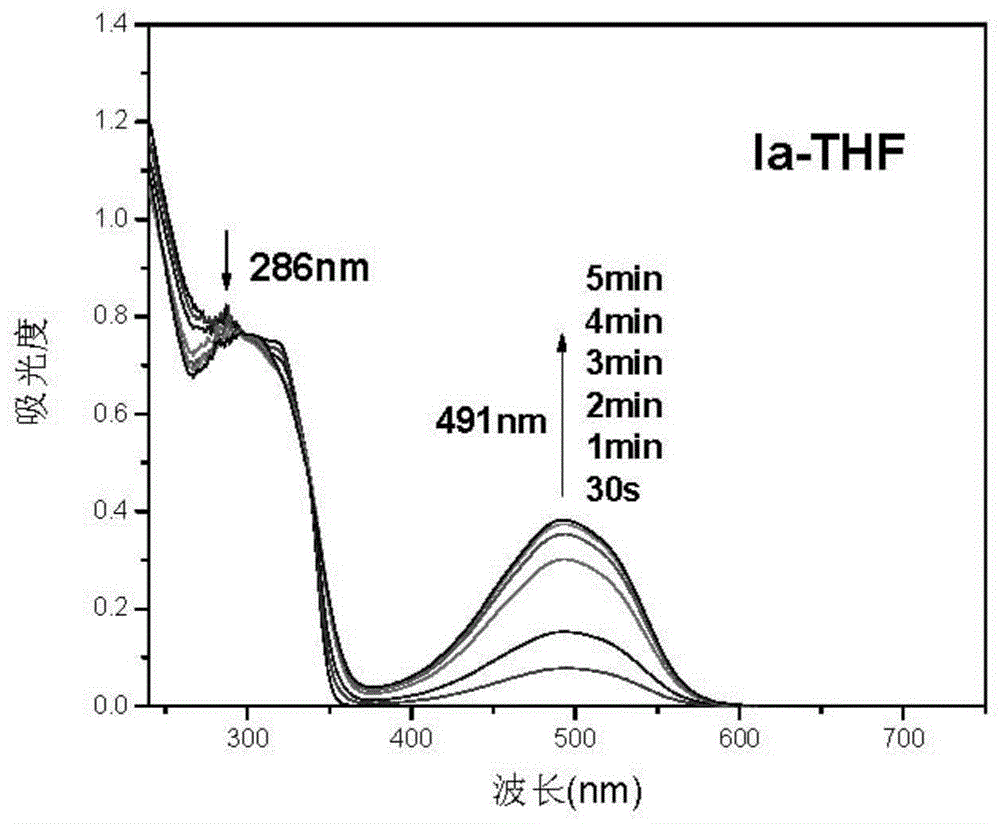
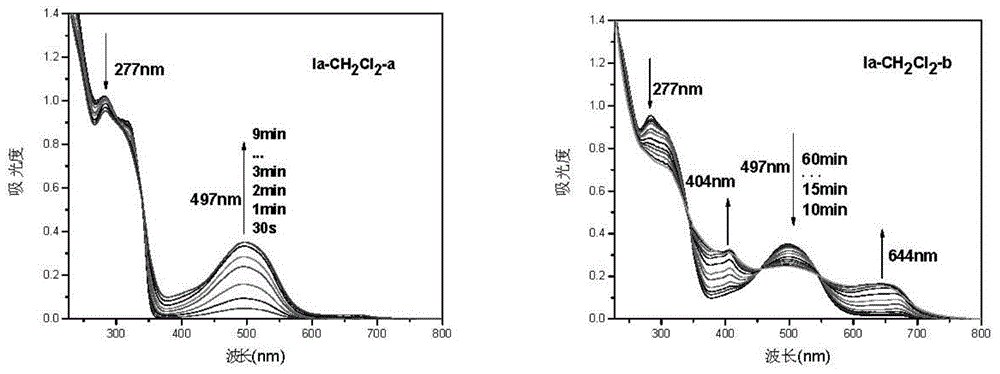
Triphyene derivative and application thereof
A derivative, triptycene technology, applied in the field of triptycene derivatives, to achieve the effects of fast light response time, good thermal stability and high quantum efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] The method for the compound shown in the preparation formula I provided by the present invention specifically comprises the following steps:
[0025] (1) In the presence (protection) of an inert gas (a gas that does not participate in the reaction, such as argon, etc.) and under light-shielding conditions, the compound shown in Formula IV and the solvent are placed in a dry round-bottomed flask, at (-15 ° C) ~-10°C), add n-BuLi cyclohexane solution to the round bottom flask, after the addition is complete, stir at room temperature for at least 45 minutes, then add trimethyl borate, and continue stirring for at least 4 hours to obtain the formula III Compound shown (no need for separation and purification, can be directly used in the next step);
[0026] (2) In the presence (protection) of an inert gas (a gas that does not participate in the reaction, such as argon, etc.), add an aqueous solution of potassium carbonate to the tetrahydrofuran (THF) solution of the corresp...
Embodiment 1
[0033] Synthesis of compounds shown in formula Ia
[0034]
[0035] Under reduced pressure, the compound shown in formula IIa (1.0mmol, 328mg) and 10mL of freshly distilled anhydrous tetrahydrofuran were placed in a dry reaction flask, and placed in an ice-salt bath Under the condition of stirring, 2Mn-BuLi (1.2mmol, 0.6mL) was added dropwise, after the addition was completed, the ice-salt bath was removed, and after stirring at room temperature for 45 minutes, trimethyl borate (1.2mmol, 0.134mL) was added, and After that, the stirring reaction was continued for 4 hours to obtain the compound represented by formula IIIa, which was directly used in the next coupling reaction without isolation.
[0036] In a two-necked flask, add 0.21 g of triiodotriptycene (0.33 mmol) shown in formula A and freshly distilled anhydrous tetrahydrofuran, then add tetrakis triphenylphosphine palladium (35 mg, 0.03 mmol), 2 mL of potassium carbonate aqueous solution (2M), vacuum, argon protecti...
Embodiment 2
[0039] Synthesis of compound shown in formula Ib
[0040]
[0041] Except that the compound shown in Formula IIa in Example 1 was replaced by the compound shown in Formula IIb, the other steps were the same as in Example 1 to obtain 123 mg of the compound shown in Formula Ib as a solid with a yield of 25.5%.
[0042] 1 H NMR (400MHz, CDCl 3 ,ppm):δ=7.54(t,J=7.0Hz,3H),7.36-7.32(m,3H),7.18-7.10(m,3H),7.04(s,3H),6.88(s,3H), 5.42(d,J=6.9Hz,2H),2.40(s,9H),2.09(s,9H),1.95(s,9H).HRMS[C 71 h 71 f 18 S 6 ]:calcd for[M+H] + :1458.3671, found 1458.3682.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com