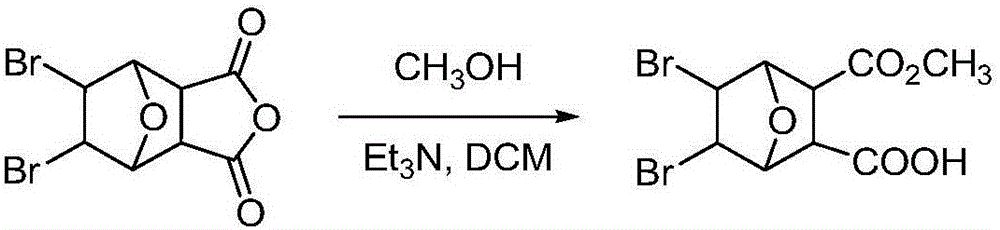
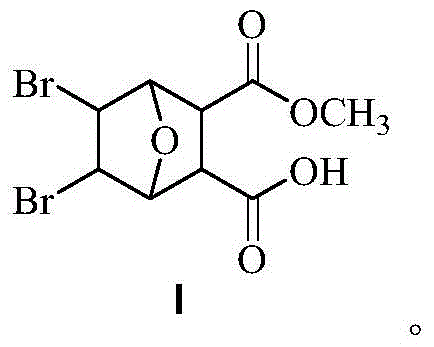
Bromonorcantharidin monoacid methyl ester and its synthesis method and application
A technology of norcantharidin and methyl monoacid can be applied in the directions of drug combination, organic chemistry, antitumor drugs, etc., can solve problems such as unreported, and achieve the effects of low cost, easy operation and implementation, and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
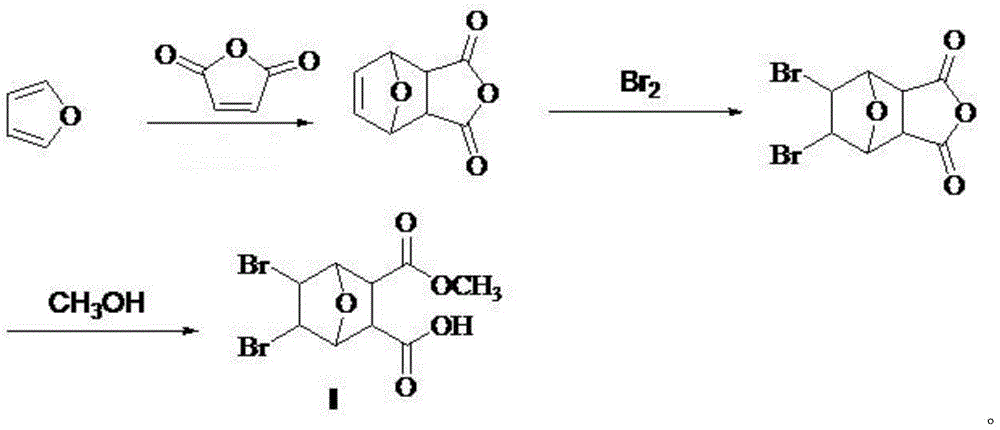
[0027] The preparation of embodiment 1 intermediate A, i.e. 5,6-dihydronorcantharidin: the reaction formula is as follows:
[0028]
[0029] Take out a certain amount of maleic anhydride from the reagent bottle, place it in a dry grinding body and grind it finely, then weigh 12.021g of the finely ground maleic anhydride with an electronic balance, put it in a dry three-necked flask, and plug it Stopper, add diethyl ether and stir, when the amount of diethyl ether is 90mL, the maleic anhydride is completely dissolved. After the maleic anhydride was completely dissolved, 13 mL of furan was slowly added through the dropping funnel for 13 minutes. The temperature was controlled to start the reaction at 38°C. After reacting for 1 h, white solids appeared in the solution, and the longer the time, the more white solids there were. After reacting for 24 hours, it was suction filtered to obtain white solid intermediate A, namely 5,6-dihydronorcantharidin. The dry weight is 17.459...
Embodiment 2
[0031] The preparation of embodiment 2 intermediate B, i.e. 5,6-dibromonorcantharidin: the reaction formula is as follows:
[0032]
[0033] Weigh 5 g of intermediate A obtained in Example 1, place it in a 250 mL two-necked flask, add 20 mL of chloroform and start stirring at room temperature until the reaction solution in the reaction system is stirred into a suspension. At this time, under stirring at room temperature, use a dropping funnel to add dropwise a mixture of 2.5 mL of chloroform and 0.5 mL of liquid bromine. After the addition is complete, rinse the dropping funnel with 2.5 mL of chloroform, and slowly add the rinse , Add the mixed solution and the rinse solution for a total of 20 minutes, and observe the phenomenon. After the reaction was completed, it was suction filtered and washed three times with carbon tetrachloride to obtain white solid intermediate B, namely 5,6-dibromonorcantharidin. The dry weight is 8.330g, and the yield is 85.35%. Melting point: 1...
Embodiment 35
[0034] Preparation of embodiment 35,6-dibromonorcantharidin monoacid methyl ester: the reaction formula is as follows:
[0035]
[0036] Weigh 0.5 g of intermediate B obtained in Example 2, place it in a round-bottomed flask, add 5 mL of anhydrous methanol, start stirring while heating to 45 ° C, and continue to slowly add 5 mL of anhydrous methanol during the stirring process. The reaction started to become turbid, and after 5 hours, it was filtered with suction to obtain a white solid, ie methyl 5,6-dibromonorcantharidin monoate. The dry weight was 472 mg, and the yield was 86%. Melting point: >240°C. Rf: 0.62 (developing solvent: petroleum ether: ethyl acetate = 10:1). 1 HNMR (DMSO-d6) δ: 12.61 (br, 1H), 4.76 (d, 2H), 4.68 (s, 2H), 3.51 (s, 3H), 3.12 (s, 2H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com