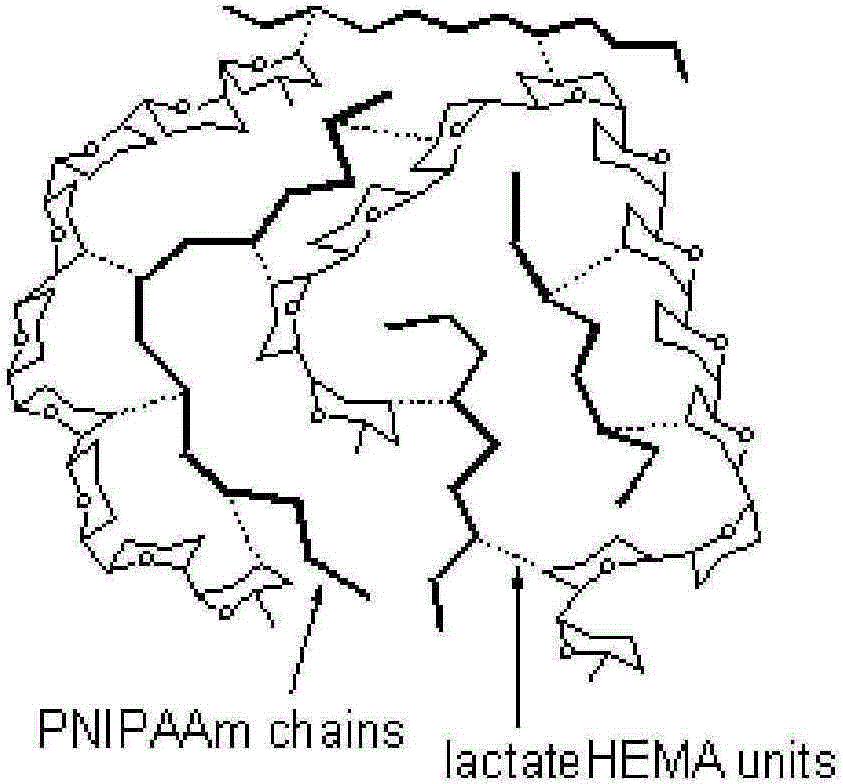
Method for synthesizing heat-sensitive and biodegradable hydrogel
A technology of biodegradation and synthesis method, applied in the field of polymer hydrogel synthesis, can solve problems such as high molar mass and inability to achieve biodegradation, and achieve the effects of simple operation, safe and reliable synthesis method, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0036] Example 1: The following steps were taken to synthesize a heat-sensitive, biodegradable hydrogel:
[0037] Weigh HEMA and L-lactide with a weight molar ratio of 2:1, and react at 110° C. to obtain HEMA-lactic acid under the catalysis of SnOct2;
[0038] At room temperature, HEMA-lactic acid was reacted with an equimolar amount of N,N'-carbonyldiimidazole (CDI) in tetrahydrofuran (THF) for 17 hours to obtain HEMA-lactateCI;
[0039] HEMA-lactateCI and dextran (molecular weight about 15kDa) were fed at a feed ratio of 1:10, catalyzed by 4-(N,N-dimethylamino)pyridine (DMAP), in dimethyl sulfoxide (DMSO ) solution at room temperature for 4 days to obtain DEXlactateHEMA macromolecule;
[0040] The mass ratio of PNIPAAM and DEXlactateHEMA macromer is 16:1, dissolved in 50% water-1% bovine serum albumin (BSA) (weight percent, pH 2), and the precursor solution is filled in a glass cover with a In the polytetrafluoroethylene model well;
[0041] The precursor s...
example 2
[0043] Example 2: The following steps were taken to synthesize a heat-sensitive, biodegradable hydrogel:
[0044] Weigh HEMA and L-lactide with a weight molar ratio of 3:1, and react at 110° C. to obtain HEMA-lactic acid under the catalysis of SnOct2;
[0045] At room temperature, HEMA-lactic acid was reacted with an equimolar amount of N,N'-carbonyldiimidazole (CDI) in tetrahydrofuran (THF) for 16 hours to obtain HEMA-lactateCI;
[0046] HEMA-lactateCI and dextran (molecular weight about 15kDa) were catalyzed by 4-(N,N-dimethylamino)pyridine (DMAP) at a molar ratio of 1:6 in dimethyl sulfoxide (DMSO) React in the solution at room temperature for 4 days to obtain DEXlactateHEMA macromolecule;
[0047] The 7:2 mass ratio of PNIPAAM and DEXlactateHEMA macromer was dissolved in 70% water-1% bovine serum albumin (BSA) (weight percent, pH 2), and the precursor solution was filled in a glass cover the Teflon model well;
[0048] The precursor solution was stirred i...
example 3
[0050] Example 3: The following steps were taken to synthesize a heat-sensitive, biodegradable hydrogel:
[0051] Weigh HEMA and L-lactide with a weight molar ratio of 4:1, and react under the catalysis of SnOct2 at 110°C to obtain HEMA-lactic acid;
[0052] At room temperature, HEMA-lactic acid was reacted with an equimolar amount of N,N'-carbonyldiimidazole (CDI) in tetrahydrofuran (THF) for 19 hours to obtain HEMA-lactateCI;
[0053] HEMA-lactateCI was added to dextran (molecular weight about 15kDa) at a molar feed ratio of 1:5, under the catalysis of 4-(N,N-dimethylamino)pyridine (DMAP), in dimethyl sulfoxide ( DMSO) solution at room temperature for 4 days to obtain DEXlactateHEMA macromolecule;
[0054] PNIPAAM and DEXlactateHEMA macromer are dissolved in 25% water-1% bovine serum albumin (BSA) (weight percent, pH 2) at a mass ratio of 2:1, and the precursor solution is filled in a belt Teflon model wells with glass covers;
[0055] The precursor solutio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com