Rapidly-degraded Cas9-ODC422-461 fusion protein and application thereof
A fusion protein and rapid degradation technology, applied in the field of molecular biology, can solve problems such as off-target effects, achieve short half-life, small side effects, and reduce non-specific cleavage toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Cas9-ODC 422–461l and Cas9-ubiquitin G75A / G76V Preparation of fusion protein
[0037] Methods as below:
[0038] Part 1: Cas9-ODC 422-461 Construction of eukaryotic expression vectors:
[0039] PEST sequences rich in proline, glutamic acid, serine, and threonine are associated with rapid protein degradation. We used PESTFind (http: / / emboss.bioinformatics.nl / cgi-bin / emboss / pestfind) to detect Cas9 protein Analysis was performed and no potential PEST sequences were found. Cas9 has a long half-life. In order to reduce its half-life. We fused the PEST sequence ODC at the C-terminus of the Cas9 protein 422–461 and Ub-G75A / G76V, constructed into the following vector, pCas9-ODC 422–461 , pCas9-ubiquitin G75A / G76V , construct pCas9-ubiquitin G75A / G76V as a comparison.
[0040] The specific construction method is as follows:
[0041] (1) Using Cas9-ODC 422–461 DNA sequence as template (SEQ ID NO.2), using primer Primer_Cas9_F: CAGCCTCCGGACTCTAGA...
Embodiment 2
[0072] Example 2 Cas9-ODC 422–461l and Cas9-ubiquitin G75A / G76V Protein expression and degradation in eukaryotic cells
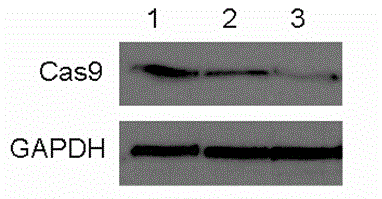
[0073] Wild-type pCas9, pCas9-ODC 422–461 and pCas9-ubiquitin G75A / G76V After transfection into 293T cells, 24 hours after transfection, they were treated with cycloheximide (50 mg / mL) for 6 hours to prevent the synthesis of new proteins. Cells were collected, lysed, and then used for Western blotting. Take 10ul of the fermentation broth supernatant and load the sample. After SDS-PAGE protein electrophoresis, the protein was transferred to PVDF membrane, combined with primary antibodies (Cas9 antibody, mouse antibody,) and HRP-labeled secondary antibody (rabbit anti-mouse,) was then developed onto film.
[0074] The result shows as figure 1 As shown, compared with wild-type Cas9, the expression of other fusion proteins is relatively low, indicating that Cas9-ODC 422–461l and Cas9-ubiquitin G75A / G76V The fusion protein is degraded and has a ...
Embodiment 3
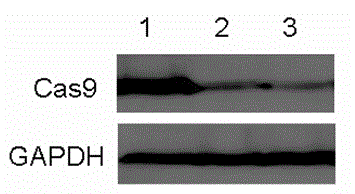
[0076] pCas9, pCas9-ODC 422–461 and pCas9-ubiquitin G75A / G76V The promoter of the plasmid was replaced with TRE, and the vector pTRE-Cas9, pTRE-Cas9-ODC was constructed 422–461 and pTRE-Cas9-ubiquitin G75A / G76V2 , the three vectors were transfected into the HEK 293 Tet-On cell line, and then screened with G418. Find expression Cas9, Cas9-ODC 422–461 and Cas9-ubiquitin G75A / G76V2 expressing cells. Tetracycline was treated for 1 hour, and after tetracycline was removed, six hours later, western blot was used to detect the expression of the three proteins, as shown in figure 2 shown. The results indicated that Cas9-ODC 422–461 and Cas9-ubiquitin G75A / G76V2 Fusion proteins have a relatively short half-life.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com