Flurbiprofen hydrogel plaster and composition thereof
A flurbiprofen and water-based gel matrix technology, applied in the field of flurbiprofen hydrogel patch, can solve problems such as late start of research, no safe and effective method found, and achieve low dosage and transdermal and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
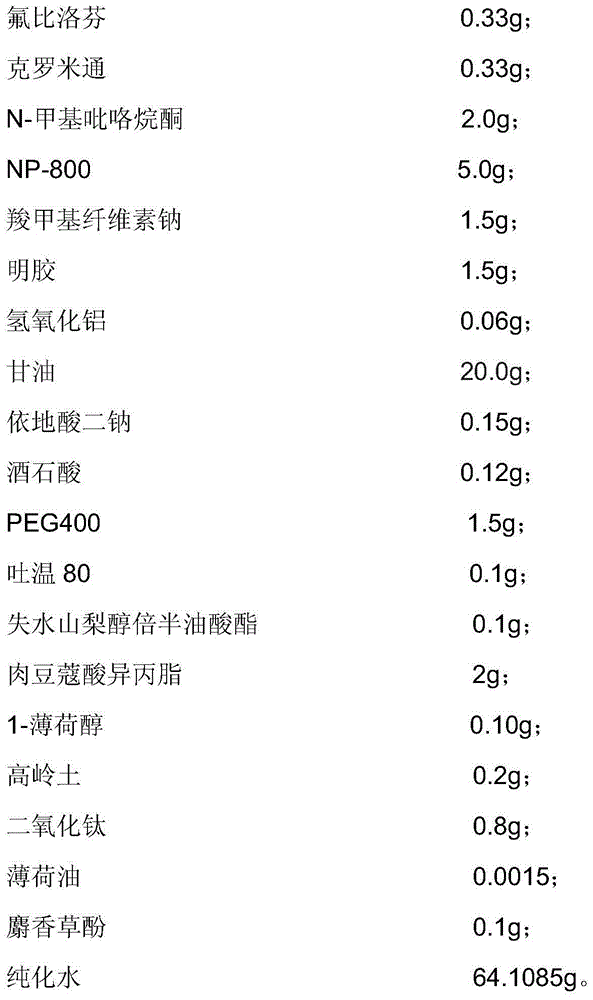
[0041] The flurbiprofen hydrogel plaster provided in this embodiment is composed of a backing, a paste and an anti-adhesive film, and the composition of the paste includes:
[0042]
[0043] Dissolve 1.5 g of sodium carboxymethyl cellulose in glycerin and partially purified water to obtain component A.
[0044] Dissolve 1.5g of gelatin in partially purified water, heat to 60°C after swelling is complete, and stir evenly until translucent colloid is obtained to obtain component B.
[0045] Dissolve 5.0 g of sodium polyacrylate partially neutralized (NP800) and 0.06 g of aluminum hydroxide in glycerin, stir and mix evenly to obtain component C.
[0046] Dissolve flurbiprofen in crotamiton and N-methylpyrrolidone in advance to make a flurbiprofen solution, then add 0.1 g of sorbitan sesquioleate, 800.1 g of Tween, and isomyristate Add 2 g of acetone, 0.0015 g of peppermint oil, 0.10 g of 1-menthol, and 1.5 g of PEG400, mix, heat at 40-50°C, and stir evenly to obtain component...
Embodiment 2
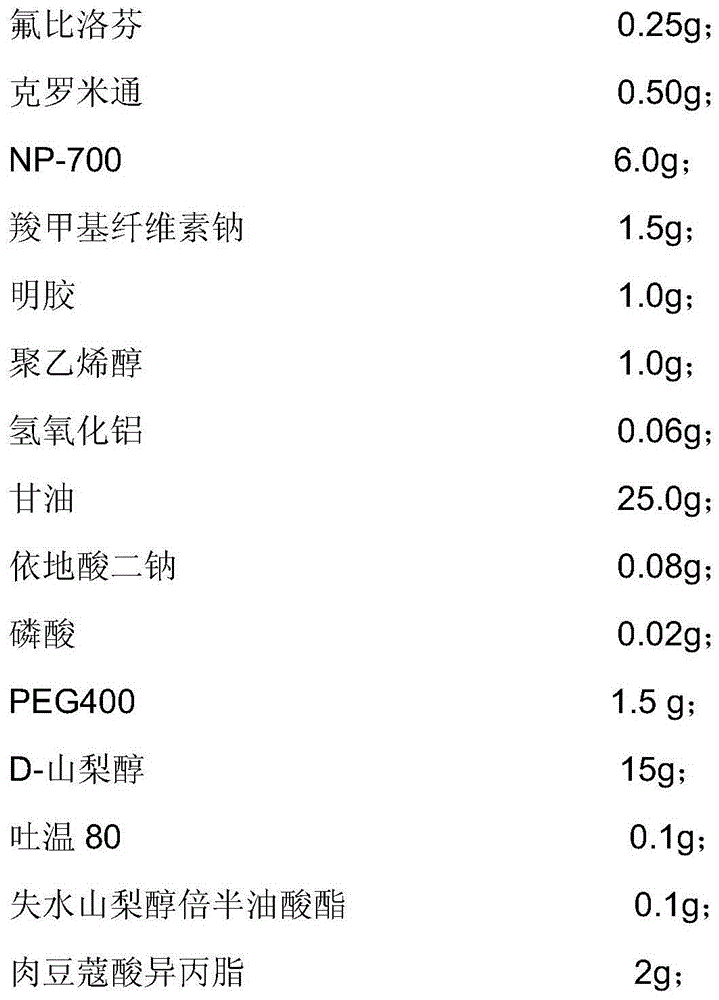
[0050] The flurbiprofen hydrogel plaster provided in this embodiment is composed of a backing, a paste and an anti-adhesive film, and the composition of the paste includes:
[0051]
[0052]
[0053] Dissolve 1.5 g of sodium carboxymethyl cellulose and 1.0 g of polyvinyl alcohol in glycerin and partially purified water to obtain component A.
[0054] Dissolve 1.0 g of gelatin in partially purified water, heat to 60°C after swelling is complete, and stir until a translucent colloid is obtained to obtain component B.
[0055] Dissolve 6.0 g of sodium polyacrylate partially neutralized (NP700) and 0.06 g of aluminum hydroxide in glycerin, stir and mix evenly to obtain component C.
[0056] Dissolve flurbiprofen in crotamiton in advance, then add sorbitan sesquioleate 0.1g, Tween 800.1g, isopropyl myristate 2g, and then add 1-menthol 0.10g , PEG400 1.5g, D-sorbitol 15g, capsaicin 0.5g, mixed, heated at 40-50°C, stirred evenly to obtain D component.
[0057] Add 0.02 g of p...
Embodiment 3
[0060] The flurbiprofen hydrogel plaster provided in this embodiment is composed of a backing, a paste and an anti-adhesive film, and the composition of the paste includes:
[0061]
[0062]
[0063] Dissolve 1.0 g of sodium carboxymethylcellulose in glycerin and partially purified water to obtain component A;
[0064] Dissolve 2.0 g of gelatin in partially purified water, heat to 60°C after swelling is complete, and stir until a translucent colloid is obtained to obtain component B.
[0065] Dissolve 8.0 g of sodium polyacrylate partially neutralized (NP600) and 0.08 g of aluminum glycolate in glycerin, stir and mix evenly to obtain component C.
[0066] Add flurbiprofen to 2.0g of PEG400, 0.0015g of peppermint oil, 0.0015g of anise oil, 0.5g of polyoxyethylene hydrogenated castor oil, and 0.08g of 1-menthol to mix, heat at 40-50°C, and stir evenly to obtain D component.
[0067] Add 0.12 g of tartaric acid into purified water to obtain component E.
[0068] Disperse...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com