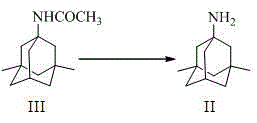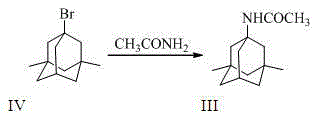Method for preparing memantine hydrochloride
A technology for memantine hydrochloride and a compound, which is applied in the fields of medicine and chemistry to achieve the effects of controlling neuron dysfunction, reducing residual solvent with high boiling point, and avoiding solvent azeotrope effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
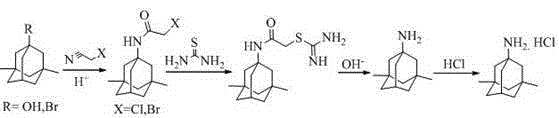
[0038] 1.1 Preparation of 1-acetylamino-3,5-dimethyladamantane (III)
[0039] Compound (IV) (100g, 0.411mol) and acetamide (121.5g, 2.057mol) were stirred and mixed, heated in an oil bath to 130°C and kept for 4~5h, then the reaction was stopped. After cooling, add 1000ml of dichloromethane and 500ml of drinking water, stir evenly, let stand, separate the liquids, extract the water phase with 500ml of dichloromethane again, and combine the organic phases. 1000ml of drinking water was washed twice, the organic phase was concentrated to dryness under reduced pressure, recrystallized with water, and dried under reduced pressure to obtain compound (III) as a white solid (GC>99.0%, yield 92%).
[0040]
[0041] 1.2 Preparation of 1-amino-3,5-dimethyladamantane (II)
[0042] Compound (III) (80g, 0.361mol) was dissolved in 400ml of n-butanol under the protection of nitrogen, 85% KOH (95.4g, 1.45mol) was added with stirring, heated to 120°C for 10~11h, and the reaction was stopp...
Embodiment 2
[0050] 2.1 Preparation of 1-acetylamino-3,5-dimethyladamantane (III)
[0051] Compound (IV) (100g, 0.411mol) and acetamide (97.2g, 1.646mol) were stirred and mixed, heated in an oil bath to 120°C and kept for 4~5h, then the reaction was stopped. After cooling, add 1000ml of dichloromethane and 500ml of drinking water, stir evenly, let stand, separate the liquids, extract the water phase with 500ml of dichloromethane again, and combine the organic phases. 1000ml of drinking water was washed twice, the organic phase was concentrated to dryness under reduced pressure, recrystallized with water, and dried under reduced pressure to obtain compound (III) as a white solid (GC>99.0%, yield 91%).
[0052]
[0053] 2.2 Preparation of 1-amino-3,5-dimethyladamantane (II)
[0054] Dissolve compound (III) (80g, 0.361mol) in 400ml of n-butanol under the protection of nitrogen, add 85% KOH (119.3g, 1.807mol) under stirring, heat the oil bath to 130°C for 10~11h, stop reaction. Cool, a...
Embodiment 3
[0058] 3.1 Preparation of 1-acetylamino-3,5-dimethyladamantane (III)
[0059] Compound (IV) (60g, 0.247mol) and acetamide (29.2g, 0.494mol) were stirred and mixed, heated in an oil bath to 125°C and kept for 6~7h, then the reaction was stopped. After cooling, add 600ml of dichloromethane and 300ml of drinking water, stir evenly, let stand, separate the liquids, extract the water phase with 300ml of dichloromethane again, and combine the organic phases. 600ml of drinking water was washed twice, the organic phase was concentrated to dryness under reduced pressure, recrystallized with water, and dried under reduced pressure to obtain compound (III) as a white solid (GC>99.0%, yield 87%).
[0060]
[0061] 3.2 Preparation of 1-amino-3,5-dimethyladamantane (II)
[0062] Dissolve compound (III) (40g, 0.181mol) in 240ml n-butanol under nitrogen protection, add 85% KOH (119.3g, 1.807mol) under stirring, heat the oil bath to 125°C for 8~9h, stop reaction. Cool, add 240ml drinki...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com