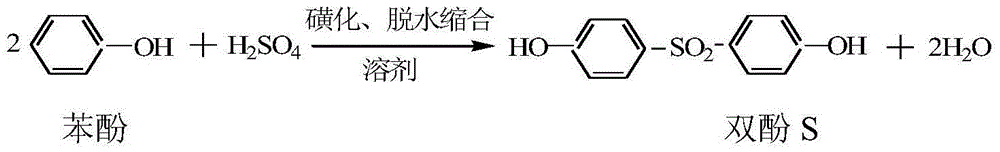
Dehydration control method for synthesis of bisphenol S
A control method and technology of synthesis reaction, applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry, etc., can solve the problems of poor control of dehydration process, low yield of bisphenol S, poor product quality, etc. Decolorization, purification and purification, the effect of improving purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add naphthalene disulfonic acid catalyst 20g, mesitylene 600g, phenol 493g in the four-necked reaction flask that reflux water divider is housed, stir and be warming up to 80 ℃, dropwise concentration 96% vitriol oil 256g (2.5 mole), and in The dropwise addition was completed in 2 hours. Then the temperature is raised rapidly to the reflux of mesitylene, and at the same time, the azeotropic dehydration is carried out by mesitylene. Theoretically, the total amount of water that needs to be removed is the sum of the water brought in by the concentrated sulfuric acid and the water generated by the reaction. Theoretically, the reaction to generate bisphenol S should produce 90mL of water, and the concentrated sulfuric acid will bring 10.2mL of water, the total is 100.2mL.
[0026] During the first 3 hours of the dehydration process, 75% of the total water, ie 75.2 mL, was removed. In the remaining 6 hours of the dehydration process, continue to remove 25% of the remaining...
Embodiment 2
[0029] Add naphthalene disulfonic acid catalyst 20g, mesitylene 600g, phenol 493g in the four-necked reaction flask that reflux water divider is housed, stir and be warming up to 80 ℃, dropwise concentration 96% vitriol oil 256g (2.5 moles), and in The dropwise addition was completed in about 2 hours. Then the temperature is raised rapidly to the reflux of mesitylene, and at the same time, the azeotropic dehydration is carried out by mesitylene. Theoretically, the total amount of water that needs to be removed is the sum of the water brought in by the concentrated sulfuric acid and the water generated by the reaction. Theoretically, the reaction to generate bisphenol S should produce 90mL of water, and the concentrated sulfuric acid will bring 10.2mL of water, the total is 100.2mL.
[0030] During the first 3 hours of the dehydration process, 85% of the total water volume, ie 85.2 mL, was removed. In the remaining 6 hours of the dehydration process, continue to remove 15% of...
Embodiment 3
[0033]Add naphthalene disulfonic acid catalyst 20g, mesitylene 600g, phenol 493g in the four-necked reaction flask that reflux water divider is housed, stir and be warming up to 80 ℃, dropwise concentration 96% vitriol oil 256g (2.5 mole), and in The dropwise addition was completed in 2 hours. Then the temperature is raised rapidly to the reflux of mesitylene, and at the same time, the azeotropic dehydration is carried out by mesitylene. Theoretically, the total amount of water that needs to be removed is the sum of the water brought in by the concentrated sulfuric acid and the water generated by the reaction. Theoretically, the reaction to generate bisphenol S should produce 90mL of water, and the concentrated sulfuric acid will bring 10.2mL of water, the total is 100.2mL.
[0034] During the first 3 hours of the dehydration process, 80% of the total water volume, ie 80.2 mL, was removed. In the remaining 6 hours of the dehydration process, continue to remove 20% of the rem...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com