Fasudil-lipoic acid dyad and application thereof
A doublet, lipoic acid technology, applied in the field of medicine of the compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the synthesis of fasudil lipoic acid
[0033] (R)-5-(1,2-Dithiolan-3-yl)-1-(4-(isoquinolin-5-ylsulfonyl)-1,4-diazepane-1 -yl)pentan-1-one Add lipoic acid (100mg) and fasudil hydrochloride (158mg) into a dry 25mL round bottom flask, add redistilled dichloromethane solution (5mL), triethylamine (0.18mL) and EDCI (140mg). The reaction solution was stirred overnight at room temperature under the protection of argon, then diluted with ethyl acetate (30 mL), and terminated with saturated aqueous ammonium chloride (3 mL). After separation, the aqueous phase was extracted with ethyl acetate (5 mL×3). The combined organic phases were washed with saturated brine (3 mL×1). in Na 2 SO 4 After drying, the solvent was removed by rotary evaporation, and the residue was purified by silica gel column chromatography (methanol / dichloromethane 1 / 30-1 / 10) to obtain a yellow viscous oil (186mg, 80%). 1HNMR (400MHz, CDCl3) δ9.36(s, 1H), 8.69-8.67(m, 1H), 8.40(d, J=6.1Hz,...
Embodiment 2
[0034] Embodiment 2: computer virtual design
[0035] The X-ray diffraction crystal form of ROCK1 was retrieved from PDB (PDB ID: 2ESM). The binding pocket between them was predicted by MOE (Molecular Operating Environment, Canada) and MOE automatic docking algorithm. Through structure-activity search, 30 preferred binding modes were found. Introduce these combinations to minimize energy and combine calculations, and finally get the best combination. The binding energy and bonding mode were calculated by MOE. From attached figure 1 It can be seen that the isoquinoline ring of Fasudil and L-F 001 can form π-π interaction with the 90 residue of valine, and the homopiperazine ring of Fasudil can interact with the 160 residue of aspartic acid. Two hydrogen bonds are formed, and after LA is inserted, the conformation of the homopiperazine ring changes, and it can no longer form hydrogen bonds with the 160 residue of aspartic acid, and the extra LA part protrudes out of the po...
Embodiment 3
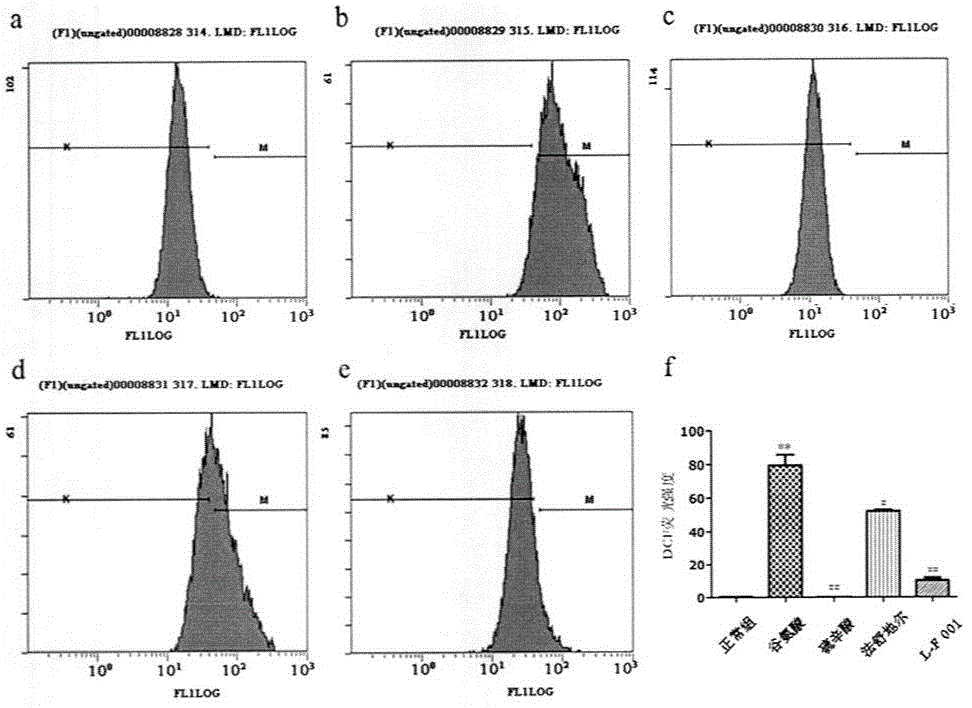
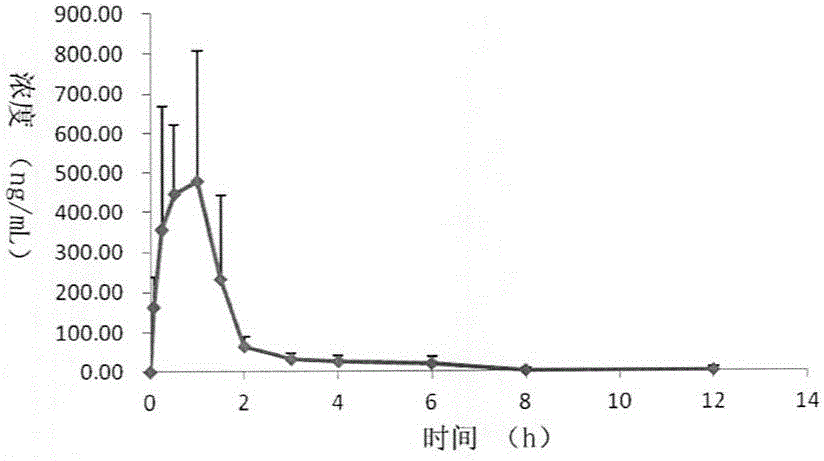
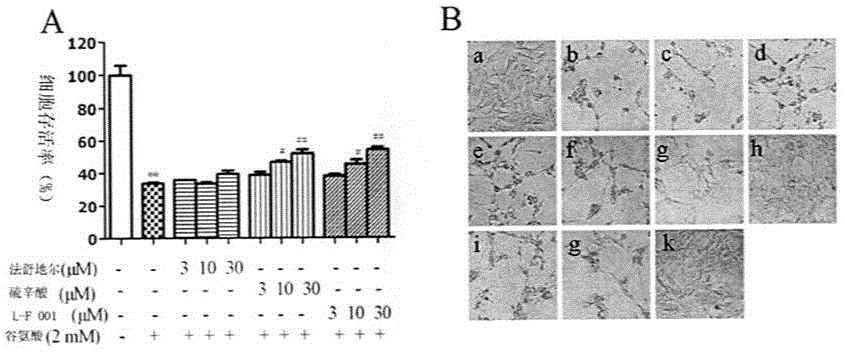
[0036] Example 3: Biological Evaluation
[0037] Inhibitory Effects of Diads on the Activities of Multiple Protein Kinases
[0038] The Z'-LYTE kinase assay kit based on fluorescence resonance energy transfer (Invitrogen, Carlsbad, CA) was used to determine the effect of compounds on ROCK1 (Rho kinase1), ROCK2, PKA (protein kinase A, PKA), PKG (protein kinase G, PKG) kinases IC 50 value. The compound to be tested was configured to 10mM, and then diluted down by 3 times step by step, and a total of 10 concentrations were set. Add 10 μL of reaction solution (containing 2 μM dissolved in 50 mM HEPES, pH7.5, 0.01% Brij-35, 10 mM MgCl 2 , 1 mM EGTA short peptide substrate, and appropriate amount of ROCK1, ROCK2, PKA, PKG) and a series of 3-fold dilutions of the test compound. The final concentration of ATP was 75 μM. After 1 h of incubation, the reaction was terminated, and the fluorescence ratio was calculated according to the instructions. The dose-response curve was fitt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com