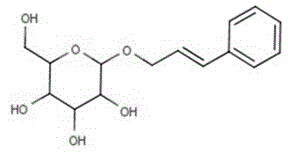
Method for catalytic synthesis of phenylallyl beta-D-glucoside from magnetic cross-linked enzyme aggregates
A technology of glucoside and cross-linking enzyme, which is applied in the field of biocatalysis, can solve the problems of high cost and achieve the effects of high added value, novel invention content and improved use efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of magnetically cross-linked β-glucosidase aggregates M-CLGAs:
[0032](1) Mix 1.351 g FeCl3, 0.685 g FeSO4 7H2O and 25 mL of deionized water at room temperature, add ammonia water until the precipitation does not increase, wash with deionized water after several centrifuges until the pH value is 7.0, and bake at 90 °C Dry for 2 h to obtain unsilanized magnetic iron oxide particles. Take 0.02 g of the above-mentioned dried and unsilanized magnetic iron oxide particles, add 2.5 mL of methanol, 100 μL of 3-aminopropyltriethoxysilane, and 25 μL of deionized water, mix well, ultrasonicate for 30 min, and then add 1.5 mL of glycerol was shaken vigorously at 90 °C for 6 h, the mixture was centrifuged and then dried at 100 °C to obtain stable silanized magnetic iron oxide particles.
[0033] (2) Add 1 mL of 2 mg / mL β-glucosidase enzyme solution prepared with pH 5.0 citric acid-phosphate buffer solution and a certain amount of magnetic iron oxide particles into a 5...
Embodiment 2
[0035] Add 100 mg of D-glucose, 220 mg of phenylpropenyl alcohol, 1.6 mL of 1,4-dioxane, and 0.4 mL of citric acid-phosphate buffer solution with a pH of 5.5 in a 10 mL ground-mouth Erlenmeyer flask to make the entire reaction system The total volume is 2 mL. Place the flask in a constant temperature water bath at 50°C and shake it well for 10 minutes, then immediately add 15 U / mL of magnetically cross-linked β-glucosidase aggregates into the flask to initiate the reaction, and cover with a rubber stopper. Finally, the flask was placed in a constant temperature shaking incubator to react at 50°C and 250rpm for 72h.
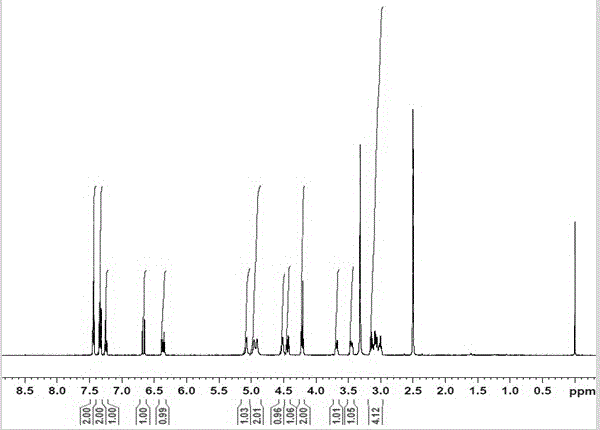
[0036] After the reaction, 100 μL was sampled, 1.9 mL of acetonitrile was added to dilute the reaction solution and ultrasonically oscillated for 20 min, centrifuged at 10,000 rpm for 5 min, the supernatant was filtered with a 0.45 μm filter membrane, and the product phenylpropenyl β-D- Glucoside was detected, and HPLC detection conditions: Waters 2996 UV detecto...
Embodiment 3
[0038] Add 100 mg of D-glucose and 120 mg of phenylpropenyl alcohol to a 10 mL conical flask, then add 1.4 mL of 1,4-dioxane, and 0.6 mL of citric acid-phosphate buffer solution with a pH of 5.5 to make the entire reaction system The total volume is 2 mL. Place the flask in a constant temperature water bath at 50°C and shake it well for 10 minutes, then immediately add 15 U / mL of magnetically cross-linked β-glucosidase aggregates into the flask to initiate the reaction, and cover with a rubber stopper. Finally, the flask was placed in a constant temperature shaking incubator to react at 50°C and 250rpm for 72h.
[0039] The product phenylpropenyl β-D-glucoside was detected by the same detection method as in Example 2, and the product concentration could reach 1.92 g / L.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com