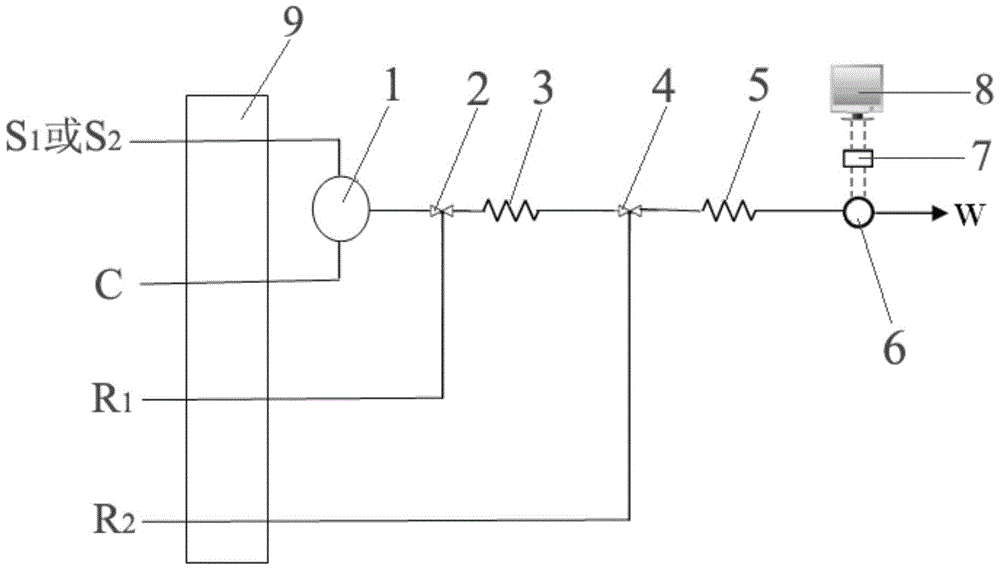
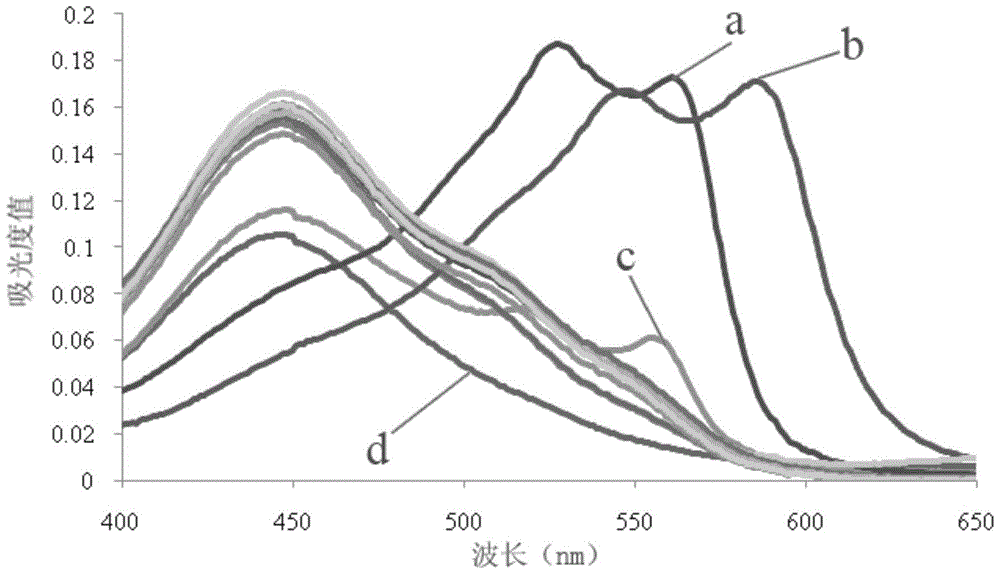
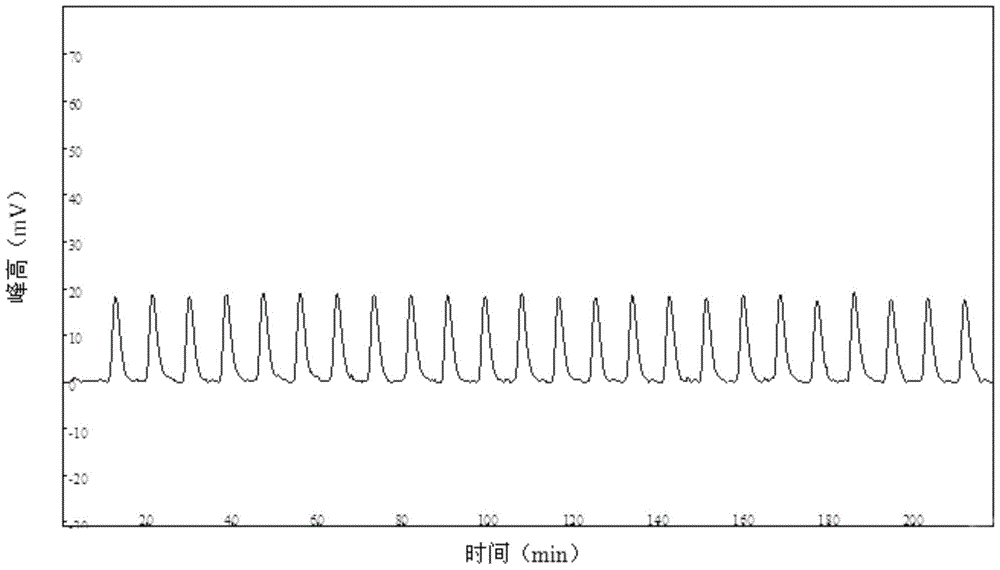
Automatic Analysis Method of Trace Divalent Nickel in Water Samples
An automatic analysis, divalent nickel technology, applied to the analysis of materials, instruments, etc., can solve the problems of poor absorbance, operator injury, high detection limit, etc., to improve the analysis speed, simplify the analysis operation, and eliminate the effect of absorbance difference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]Since the method of the present invention does not use pretreatment operations such as organic solvent extraction, some ions in the environmental water sample may interfere with the measurement of Ni(II). In this embodiment, first understand whether the interfering ions will interfere with the measurement of Ni(II) .
[0030] 1. Chromogenic solution R 1 preparation
[0031] Take 9mL of cetyltrimethylammonium bromide (CTMAB) aqueous solution with a concentration of 0.01g / L, 15mL of NH with pH=10 3 -NH 4 Cl buffer solution, 6 mL of 2-(5-bromo-2-pyridylazo)-5-diethylaminophenol (5-Br-PADAP) with a concentration of 2×10 -4 g / L 5-Br-PADAP ethanol solution, 3 mL of 0.25 g / L triethanolamine (TEA) aqueous solution, 5 mL of 0.1 g / L potassium sodium tartrate aqueous solution were added to a 50 mL volumetric flask, and deionized water was added Set the volume to the mark, and ultrasonically treat for 30 minutes to obtain the chromogenic solution R 1 , in the chromogenic soluti...
Embodiment 2
[0042] In this embodiment, the standard sample is tested to investigate the precision of the method of the present invention. The steps are as follows:
[0043] 1. Preparation of standard samples
[0044] (1) Weigh 0.4785g NiSO 4 ·7H 2 O in a beaker, dissolved in deionized water, then added nitric acid to a final concentration of 5mmol / L, then distilled to a 100mL volumetric flask with deionized water, refrigerated in a refrigerator for later use, and the solution was calibrated as Ni(II) ) concentration is the mother liquor of 1g / L;
[0045] (2) Dilute the mother liquor with simulated environmental water (20mg / L Ca 2+ , 10mg / L Mg 2+ , 100mg / L HCO 3 - ), dilute with nitric acid until the final concentration of nitric acid is 5 mmol / L, and prepare a standard sample with a Ni(II) concentration of 25 μg / L.
[0046] 2. Chromogenic solution R 1 preparation
[0047] Take 9mL CTMAB concentration and be the CTMAB aqueous solution of 0.01g / L, 15mL the NH of pH=10 3 -NH 4 Cl...
Embodiment 3
[0057] In this embodiment, the tested sample is simulated environmental water plus Ni(II) standard sample. Its analysis steps are as follows:
[0058] 1. Preparation of standard samples
[0059] (1) Weigh 0.4785g NiSO 4 ·7H 2 O in a beaker, dissolved in deionized water, then added nitric acid to a final concentration of 5mmol / L, then distilled to a 100mL volumetric flask with deionized water, refrigerated in a refrigerator for later use, and the solution was calibrated as Ni(II) ) concentration is the mother liquor of 1g / L;
[0060] (2) Prepare a series of standard samples: Dilute the mother liquor prepared in step (1) with deionized water, add nitric acid until the final concentration of nitric acid is 5 mmol / L, and prepare 1# to 15# standard samples. Ni(Ⅱ) in each standard sample ) concentrations are 5μg / L, 10μg / L, 25μg / L, 50μg / L, 100μg / L, 125μg / L, 150μg / L, 175μg / L, 200μg / L, 225μg / L, 250μg / L, 275μg / L, 300μg / L, 350μg / L, 400μg / L, and the concentration of nitric acid in 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com