Preparation method for hydrocracking catalyst containing heteropoly acid
A hydrocracking and catalyst technology, applied in physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, chemical instruments and methods, etc., can solve the problem that hydrogenation activity and cracking cannot be well coordinated , Macromolecular compounds cannot pass through the pores of the catalyst, and the distribution of hydrogenation active metals is not easy to control, etc., to achieve good performance, high performance, and good product quality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
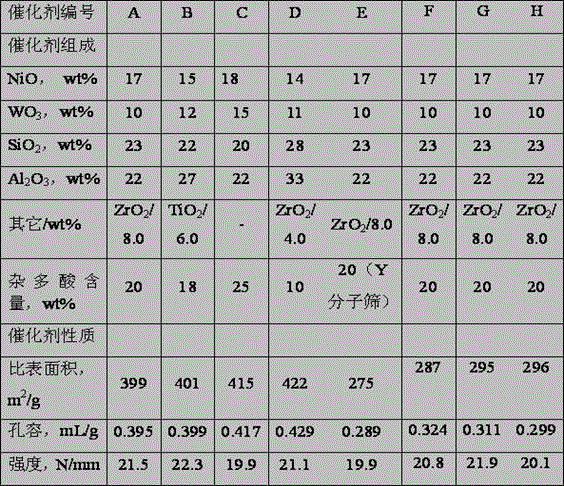
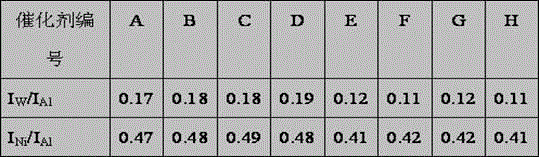
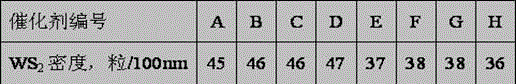
[0035] 550 ml of nickel chloride solution containing NiO of 50 g / L, containing Al 2 o 3 450 ml of 130g / L aluminum chloride solution, containing ZrO 2 130 milliliters of 110 g / L zirconium oxychloride solution was mixed in solution tank 1, diluted with 1000 milliliters of clean water, and configured as solution A. will contain WO 3 980ml of 90g / L ammonium metatungstate and containing Al 2 o 3 420 milliliters of 130g / L aluminum chloride solution, mixed in the solution tank 2, add 1000 milliliters of clean water for dilution, add 620 milliliters of SiO containing 2 100g / L dilute water glass solution, configure solution B. Add ammonia water to solution A under stirring, keep the gel forming temperature at 50°C, control the pH value at 7.6 at the end, control the gel forming time at 60 minutes, and generate slurry I containing nickel and aluminum precipitates. Add 1000mL of clean water into the reaction tank, add ammonia water and solution B into the reaction tank concurrent...
Embodiment 2
[0037] According to the method of embodiment 1, press the component content ratio of catalyst B in table 1, add nickel nitrate, titanium chloride, aluminum nitrate solution in dissolving tank 1, prepare working solution A, add nitric acid in dissolving tank 2 Aluminum, ammonium metatungstate and dilute water glass prepare working solution B, wherein dilute water glass contains SiO 2 480 ml of dilute water glass solution of 110 g / L. Add ammonia water to solution A under stirring, keep the gel forming temperature at 40°C, control the pH value at the end of the solution at 7.8, and control the gel forming time at 50 minutes to generate slurry I containing nickel and aluminum precipitates. Add 1000mL of clean water into the reaction tank, add ammonia water and solution B into the reaction tank concurrently, keep the gelling temperature at 50°C, and control the pH value during the gelling reaction process at 7.6, and control the gelling time at 50 minutes. A slurry II containing t...
Embodiment 3
[0039] According to the method of embodiment 1, press the component content ratio of catalyst C in table 1, add nickel chloride, aluminum chloride solution in dissolving tank 1, prepare working solution A, add aluminum chloride in dissolving tank 2 , ammonium molybdate, and dilute water glass to prepare working solution B, wherein the dilute water glass contains SiO 2 540 ml of 100 g / L dilute water glass solution. Add ammonia water to solution A under stirring, keep the gel forming temperature at 60°C, control the pH value at 8.0 at the end, control the gel forming time at 50 minutes, and generate slurry I containing nickel and aluminum precipitates. Add 1200mL of clean water into the reaction tank, add ammonia water and solution B into the reaction tank concurrently, keep the gelling temperature at 65°C, and control the pH value at 8.0 during the gelling reaction process, and control the gelling time at 60 minutes , generating slurry II containing tungsten, silicon, and alum...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com