1,3,5-tris(4-aminophenylmercapto)benzene as well as preparation method and application thereof
A kind of aminophenylthio group, aminothiophenol technology, applied in the field of 1,3,5-triphenyl and its preparation, can solve the problems of the improvement of refractive index, few reports and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] 0.036mol (6.534g) 1,3,5-trichlorobenzene, 0.108mol (13.5g) 4-aminothiophenol, 0.9mol (87mL) N-methylpyrrolidone 1, 0.072mol (9.936g) anhydrous Potassium carbonate and 0.288mol (30mL) toluene were added to the reactor equipped with a water tank and a reflux condenser. Under mechanical stirring and nitrogen protection, the toluene was evaporated after heating to reflux with water for 4 hours, and the temperature was raised to 160°C to continue the reaction. After 12 hours, after cooling down to room temperature, discharge the material in deionized water, and wash 3 times with deionized water until the filtrate is colorless, then wash 2 times with ethanol, and dry in vacuum at 80°C for 4 to 8 hours to obtain 1,3,5 - Tris(4-aminophenylthio)benzene.
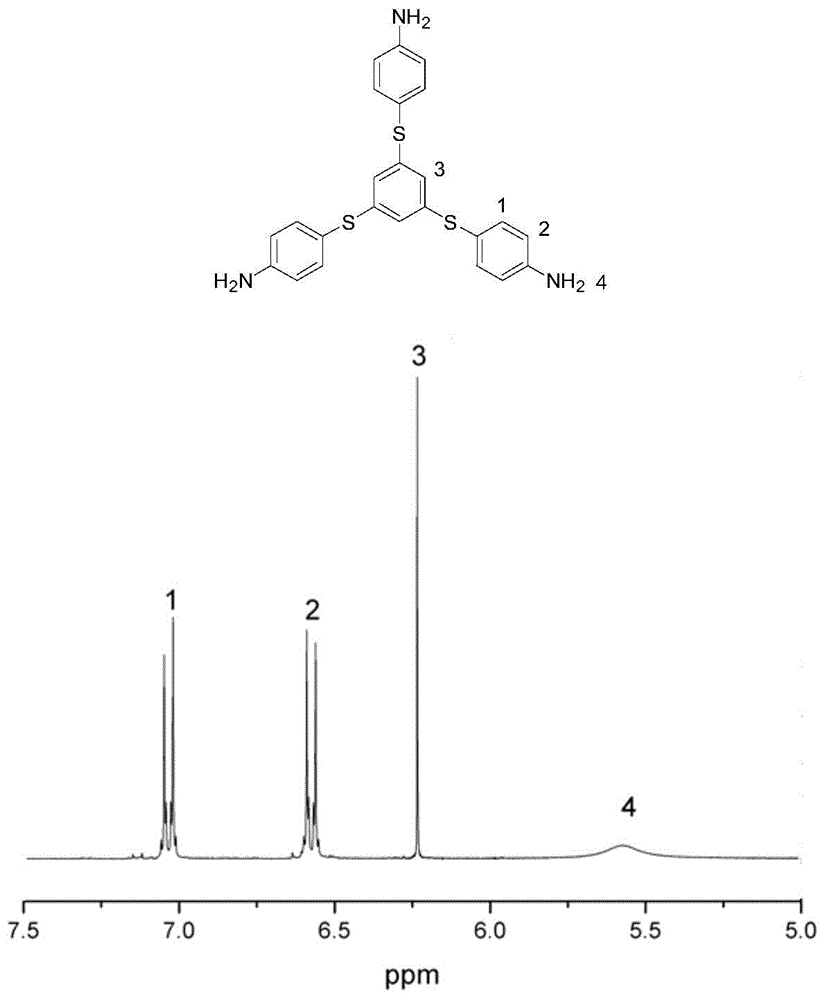
[0053] figure 1 The 300MHz test hydrogen spectrum of the monomer is given, and the chemical shifts of 1, 2, 3, and 4 marked in the figure correspond to the hydrogen on the benzene ring and the amine group respectively, and the...
Embodiment 2
[0057] Change the amount of 4-aminothiophenol to 0.1134mol (14.175g), the amount of N-methylpyrrolidone 1 to 1.08mol (104mL), the amount of potassium carbonate to 0.108mol (14.904g), and the amount of toluene Change it to 0.36mol (38mL), change the time of heating and refluxing with water to 6 hours, change the reaction temperature to 170°C, change the reaction time to 16 hours, change the number of deionized water washings to 4 times, and the number of ethanol washings to 3 times, Example 1 was repeated.
[0058] The characterization results are similar to Example 1.
Embodiment 3
[0060] Dissolve 2mmol (0.888g) of hexafluorodianhydride monomer in 0.08mol (7.73mL) of N-methylpyrrolidone 2 to obtain reaction solution 1; base) benzene was dissolved in 0.08mol (7.73mL) N-methylpyrrolidone 3 to obtain a reaction solution 2; at room temperature, the reaction solution 2 was added dropwise to the reaction solution 1 within 2 hours; during the polymerization process The viscosity of the medium solution gradually increased, and 0.2mol (19.32mL) of N-methylpyrrolidone 4 was added. After 8 hours of reaction, a polyamic acid solution was obtained, and the polyamic acid solution was cast onto a glass plate, and the temperature was raised as follows Steps for imidization: 60°C for 2 hours, 80°C for 2 hours, 100°C for 1 hour, 150°C for 1 hour, 200°C for 1 hour, and 250°C for 1 hour. After cooling down to room temperature naturally, take out the glass plate and place it in deionized water , A2+B3 type hyperbranched polyimide terminated by acid anhydride was obtained aft...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com