Preparation method and application for novel chalcone derivative containing quinazoline thioether
A kind of technology of oxazoline sulfide and quinazoline, applied in the field of novel chalcone derivatives containing quinazoline sulfide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
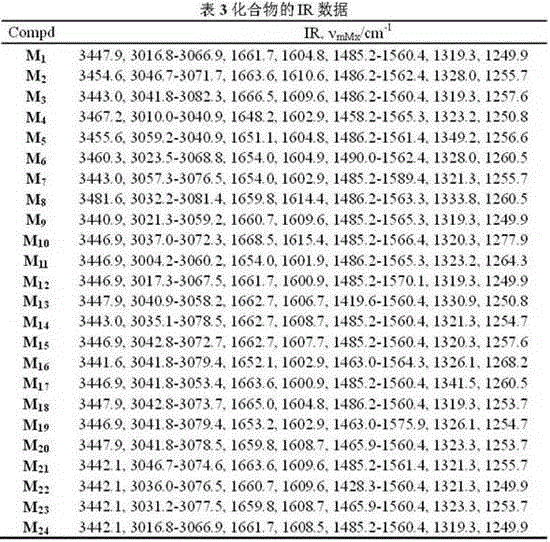
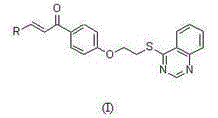
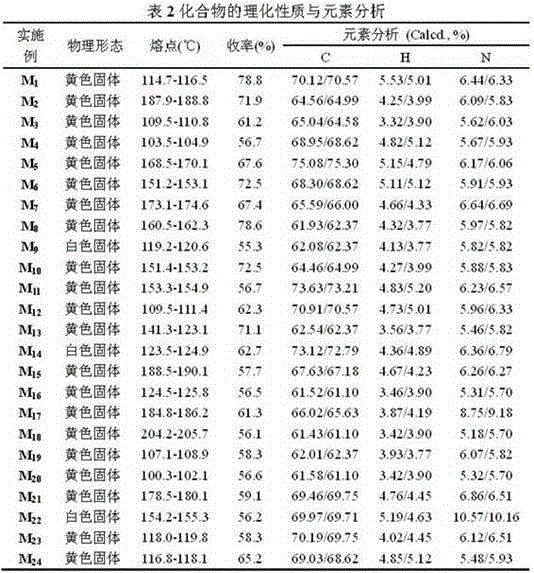
[0053] Embodiment one: (E)-3-(2-methoxyphenyl)-1-(4-(2-(quinazolin-4-ylthio)ethoxy)phenyl)prop-2-ene-1- Ketone (compound number is M 1 )Synthesis:
[0054] (1) Synthesis of intermediate 4-chloroquinazoline
[0055]Add 11.5 g (83.83 mmol) of anthranilic acid and 15.1 g (335.41 mmol) of formamide into a 100 mL round bottom flask, mix and heat to 135~145 °C, and react for 5 h. After the reaction, add 100 mL of water and cool to Add a large amount of water at 60°C, stir for 30 min, cool to room temperature, and filter with suction to obtain a light brown solid, which is recrystallized in absolute ethanol to obtain a white flocculent solid, which is quinazolin-4-one; Add quinazolin-4-one (36.32mmol), thionyl chloride (37 mL), 1,2-dichloroethane (17 mL) and DMF (1 mL) into a mL round bottom flask, reflux for 4.5 h, After the reaction is complete, evaporate most of the solvent, cool to room temperature, add 30 mL of chloroform to the residue and pour it into water together, and ...
Embodiment 2
[0064] Embodiment two: (E)-1-(4-(2-(quinazolin-4-ylthio)ethoxy)phenyl)-3-(4-(trifluoromethyl)phenyl)prop-2-ene -1-ketone (the compound number is M 2 )Synthesis:
[0065] (1) Synthesis of intermediate 4-chloroquinazoline: synthetic steps and process conditions are the same as embodiment one (1);
[0066] (2) Synthesis of intermediate 4-mercaptoquinazoline: synthetic steps and process conditions are the same as embodiment one (2);
[0067] (3) Synthesis of intermediate (E)-1-(4-hydroxyphenyl)-3-(4-(trifluoromethyl)phenyl)prop-2-en-1-one: synthesis steps and process conditions Like embodiment one (3), the difference is that 4-trifluoromethylbenzaldehyde is a raw material;
[0068] (4) Intermediate (E)-1-(4-(2-bromoethoxy)phenyl)-3-(4-(trifluoromethyl)phenyl)prop-2-en-1-one Synthesis: Synthetic steps and process conditions are as embodiment one (4);
[0069] (5) Target product (E)-1-(4-(2-(quinazolin-4-ylthio)ethoxy)phenyl)-3-(4-(trifluoromethyl)phenyl) Synthesis of prop-2...
Embodiment 3
[0070] Embodiment three: (E)-3-(2-Chloro-6-fluorophenyl)-1-(4-(2-(quinazolin-4-ylthio)ethoxy)phenyl)prop-2-ene- 1-keto (compound number is M 3 )Synthesis:
[0071] (1) Synthesis of intermediate 4-chloroquinazoline: synthetic steps and process conditions are the same as embodiment one (1);
[0072] (2) Synthesis of intermediate 4-mercaptoquinazoline: synthetic steps and process conditions are the same as embodiment one (2);
[0073] (3) Synthesis of intermediate (E)-1-(4-hydroxyphenyl)-3-(2-chloro-6-fluorophenyl)prop-2-en-1-one: the synthesis steps and process conditions are the same As in embodiment one (3), the difference is that 2-chloro-6-fluorobenzaldehyde is a raw material;
[0074] (4) Synthesis of intermediate (E)-1-(4-(2-bromoethoxy)phenyl)-3-(2-chloro-6-fluorophenyl)prop-2-en-1-one : synthetic steps and processing conditions are as embodiment one (4);
[0075] (5) Target product (E)-3-(2-chloro-6-fluorophenyl)-1-(4-(2-(quinazolin-4-ylthio)ethoxy)phenyl)propane ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com