Specific fluorescent probe for glucuronyl transferase UGT1A1 and application thereof
A glucuronic acid and fluorescent probe technology is applied in the field of specific fluorescent probe substrates for glucuronyltransferase UGT1A1, which can solve the problems of low detection sensitivity, low single-enzyme selectivity, poor chemical stability, etc. The detection cost is low, the synthesis process is simple and feasible, and the effect of sensitive detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
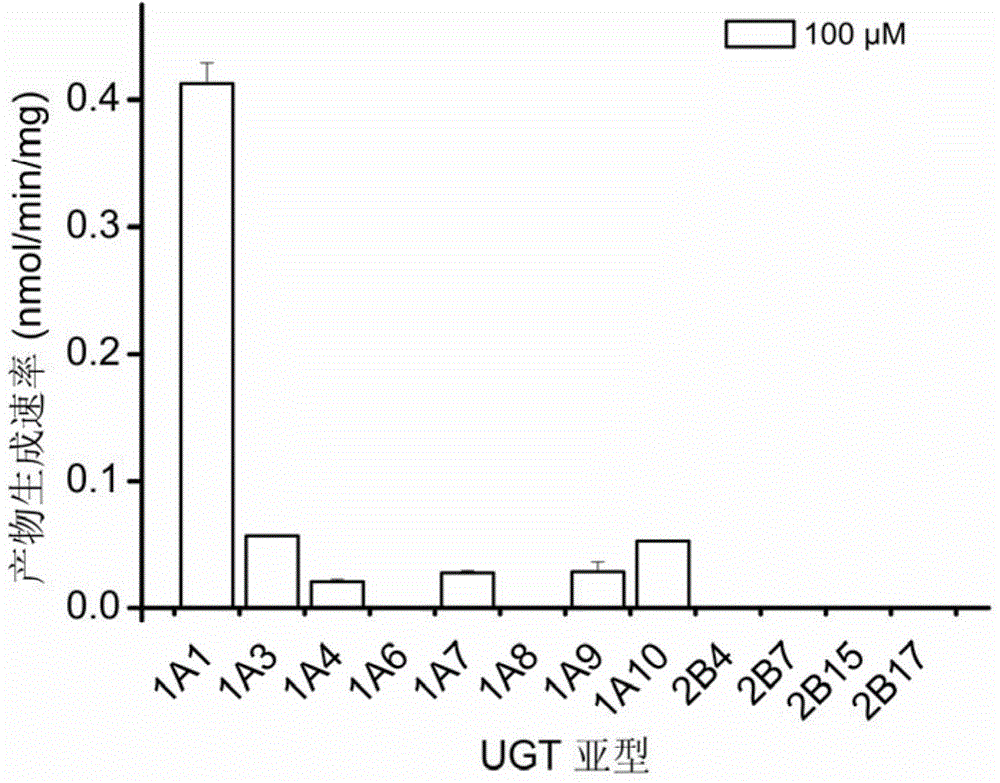
[0028] Embodiment 1. In vitro determination of the selectivity of human recombinant UGT single enzyme
[0029] (1) Prepare 95 μL UGT metabolic reaction system in advance, including Tris-HCl buffer solution (50mM) at pH 7.4, each single enzyme of recombinant human UGT (0.1mg / mL), the final substrate concentration is 100μM, shake at 37°C Pre-incubation for 3 minutes;
[0030] (2) Add 5 μL of UDPGA with a concentration of 40 mM (final concentration 2 mM) to the reaction system to initiate the reaction;
[0031] (3) After 60 minutes, add 100 μL of glacial acetonitrile, shake vigorously, and terminate the reaction;
[0032] (4) Use a high-speed refrigerated centrifuge to centrifuge at 4°C and 20,000×g for 20 minutes at high speed, take the supernatant, and perform fluorescence detection (Ex=460nm, Em=612nm); selectivity of recombinant human UGT1A1 enzyme The highest is about 8 times that of other single enzymes ( figure 1 ).
Embodiment 2
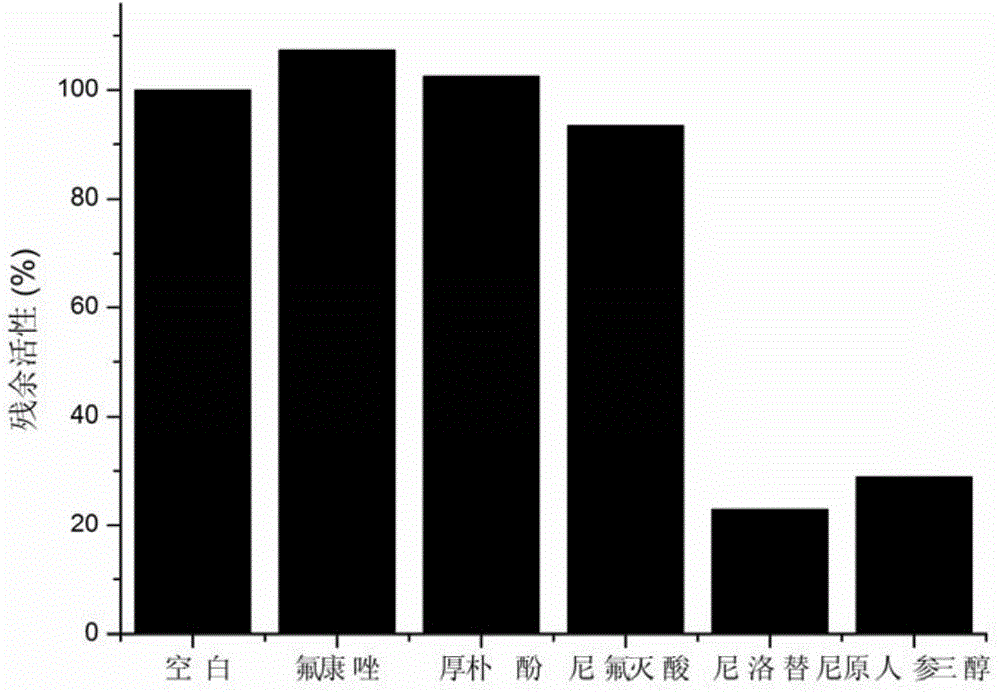
[0033] Example 2. Inhibition experiment of UGT1A1 in human liver microsomes in vitro
[0034] (1) Prepare 190 μL human liver microsomes and UGT1A1 metabolic reaction system in advance, including Tris-HCl buffer (50mM) at pH 7.4, human liver microsomes (0.25mg / mL), UGT1A1 (0.1mg / mL), substrate The final concentration is 10 μM, add 10 μM niflumic acid, 10 μM nilotinib and 500 μM fluconazole respectively, and shake for 3 minutes at 37°C;
[0035] (2) Add 10 μL of UDPGA with a concentration of 40 mM to the reaction system to initiate the reaction;
[0036] (3) After 30 minutes, add 200 μL of glacial acetonitrile, shake vigorously, and terminate the reaction;
[0037](4) Use a high-speed refrigerated centrifuge under the condition of 4°C and 20,000×g, after high-speed centrifugation for 20 minutes, take the supernatant, and perform fluorescence detection (Ex=460nm, Em=612nm); Suppress results such as figure 2 .
Embodiment 3
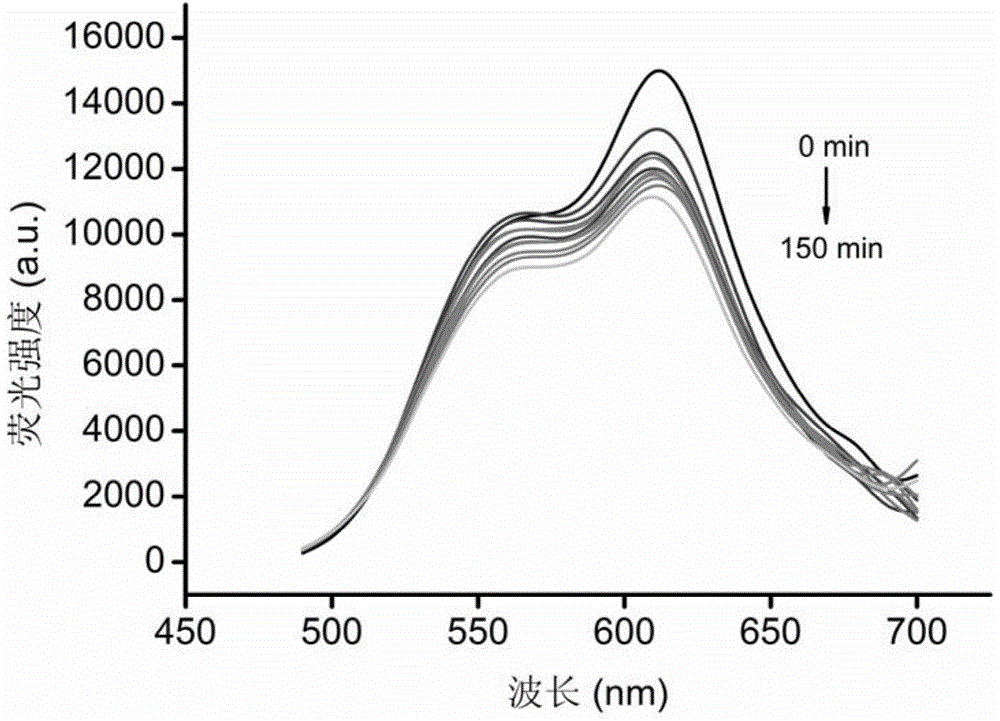
[0038] Example 3 The linear incubation time in the recombinant single enzyme UGT1A1
[0039] (1) Prepare 95 μL UGT metabolic reaction system in advance, including Tris-HCl buffer solution (50 mM) at pH 7.4, recombinant human UGT1A1 single enzyme (0.1 mg / mL), and the final substrate concentration is 10 μM. Incubate for 3 minutes;
[0040] (2) Add 5 μL of UDPGA with a final concentration of 40 mM (final concentration 2 mM) to the reaction system to initiate the reaction;
[0041] (3) Fluorescence scanning detection (Ex=460nm, Em=610nm) is carried out every 15 minutes; Calculate the linear reaction time of recombinant human UGT1A1 enzyme (see image 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com