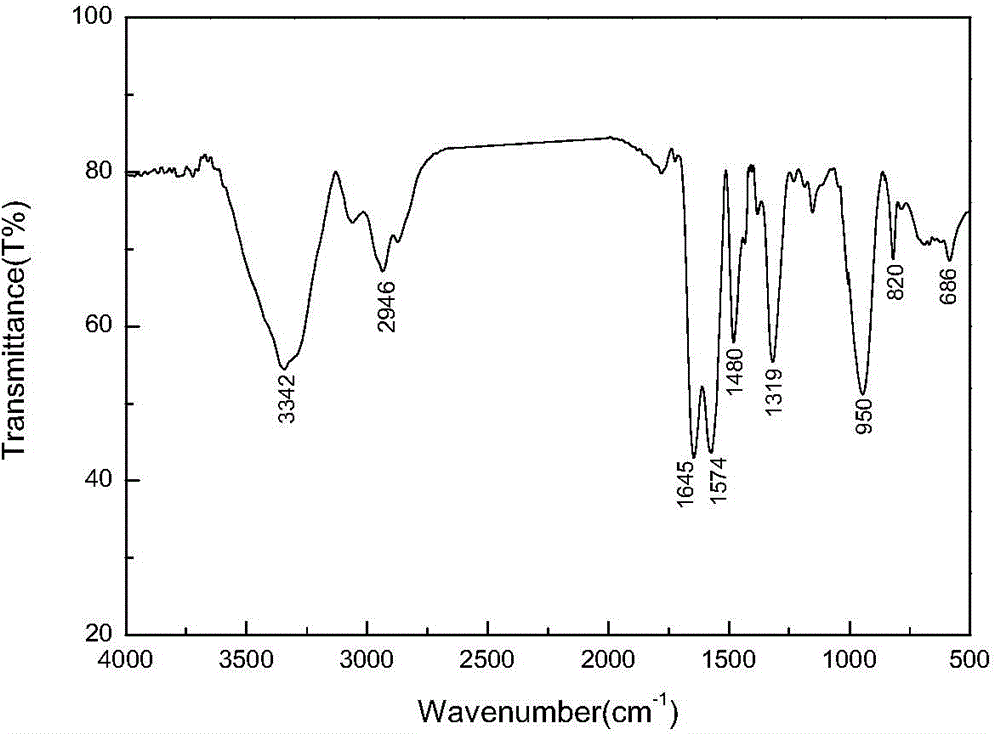
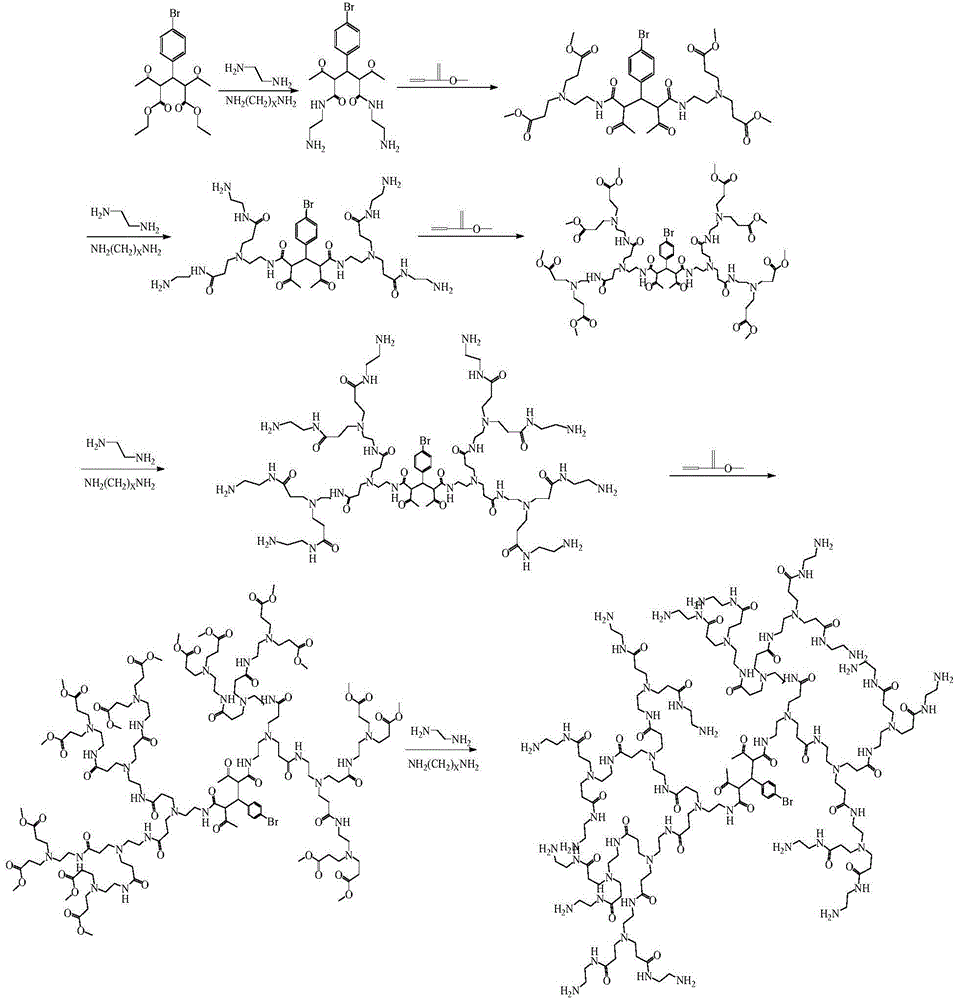
Resin framework monomer taking 2,4-diacetyl-3-(4-bromophenyl)ethyl glutarate as core as well as preparation method and application of resin framework monomer
A technology of diethyl glutarate and diacetyl, which is applied in the field of dendritic skeleton monomers and its preparation, can solve the problems of limited salt resistance, shear resistance and temperature resistance, and achieve improved salt resistance and high resistance to molecular movement , prevent chain curling effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: The preparation of rigid small molecule reaction core comprises the following steps:
[0044] Weigh 1 gram of 2,4-diacetyl-3-(4-bromophenyl) diethyl glutarate and dissolve it in 4.4 grams of ethylenediamine, wherein ethylenediamine is used both as a solvent and as a reactant, The molar ratio of 2,4-diacetyl-3-(4-bromophenyl)glutaric acid diethyl ester to ethylenediamine participating in the reaction is 1:2.5; avoid light and use a 2.5cm long rotor in an ice-water bath React on a magnetic stirrer for 24 to 25 hours. After the reaction is over, distill with a rotary evaporator, then add 15 grams of methanol, and rotate for several times to obtain a bright yellow solid, which is the reaction nucleus.
Embodiment 2
[0045] Embodiment 2: adopt the reaction core that embodiment 1 makes to carry out follow-up reaction, concrete steps are as follows:
[0046] Dissolve the bright yellow solid reaction nucleus with methanol as a solvent, add methyl acrylate dropwise with a constant pressure separatory funnel, and react with a magnetic stirrer at room temperature for 24 to 25 hours. pressure distillation, and add methanol for multiple rotary evaporation to obtain a yellow solid, i.e. the 0.5 generation product, wherein the molar ratio of the reaction nucleus to methyl acrylate is 1.5:3;
[0047] The obtained 0.5 generation product continues to be fully dissolved with methanol as a solvent, and ethylenediamine is added dropwise with a constant pressure separatory funnel, and reacted with a magnetic stirrer at room temperature for 24 to 25 hours. Distillation, and adding methanol for multiple rotary evaporation to obtain the 1st generation yellow solid product; wherein the molar ratio of the 0.5th...
Embodiment 3
[0054] Embodiment 3: adopt the reaction core that embodiment 1 makes to carry out follow-up reaction, concrete steps are as follows:
[0055] Dissolve the bright yellow solid reaction nucleus with methanol as a solvent, add methyl acrylate dropwise with a constant pressure separatory funnel, and react with a magnetic stirrer at room temperature for 24 to 25 hours. pressure distillation, and add methanol for multiple rotary evaporation to obtain a yellow solid, which is a 0.5-generation product, wherein the molar ratio of the reaction nucleus to methyl acrylate is 1:4.5;
[0056] The obtained 0.5 generation product continues to be fully dissolved with methanol as a solvent, and ethylenediamine is added dropwise with a constant pressure separatory funnel, and reacted with a magnetic stirrer at room temperature for 24 to 25 hours. Distillation, and adding methanol for multiple rotary evaporation to obtain the 1st generation yellow solid product, wherein the molar ratio of the 0.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com