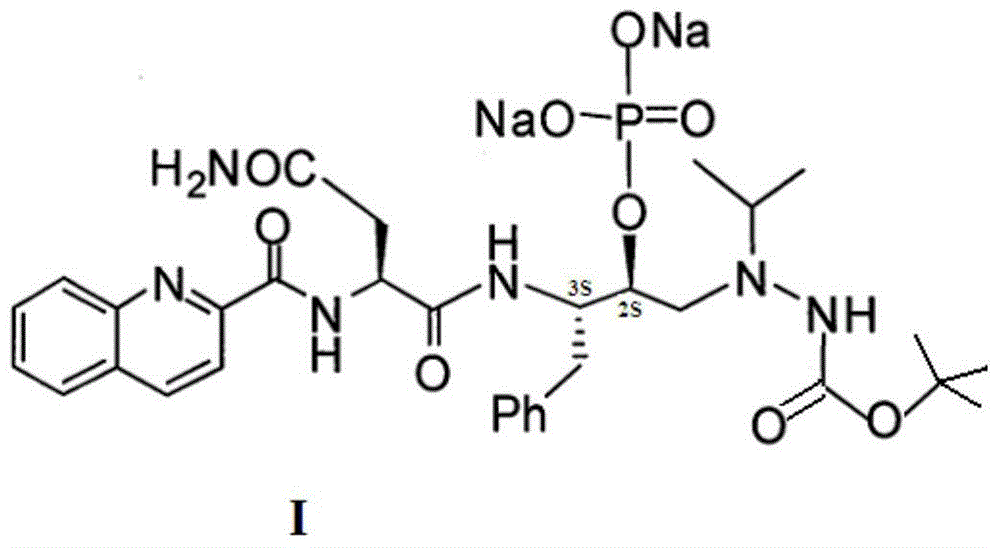
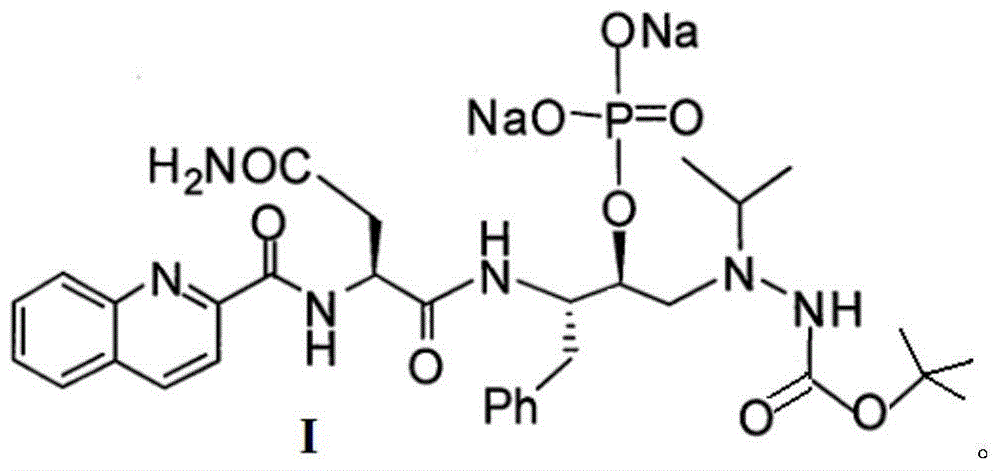
Antiviral medicine and medicine composition thereof
A composition and drug technology, applied in the direction of antiviral agents, phosphorus organic compounds, pharmaceutical formulations, etc., can solve the problems of weakened control ability of preparations, and achieve significant pharmaceutical effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0182] Embodiment 1: preparation tert-butyl 3-isopropyl-[(2R,3S)-2-hydroxy-3-(phenylmethoxycarbonyl)amino-4-phenylbutyl]carbazate
[0183] Step A: Preparation tert-Butyl 3-isopropylcarbazinate
[0184] The title compound can be prepared by the method of Dutta et al. (J.C: S.Perkin / 1975, 1712-1720) or by mixing 13.2 g (0.1 mol) tert-butyl carbazate and 6 g (0.103 mol) of acetone and 12.5g (0.1mol) of anhydrous magnesium sulfate in 100ml of dichloromethane was stirred for 12 hours, after the desiccant was removed by filtration, the filtrate was evaporated to dryness under reduced pressure, and after crystallization from cyclohexane, 16.9g (98% yield) The corresponding hydrazone has a melting point of 104-105°C. In a nitrogen atmosphere under a greenhouse, add 12ml (0.094mol) trimethylchlorosilane to a suspension of 2.04g (0.094mol) lithium borohydride in 100ml anhydrous tetrahydrofuran, and after 30 minutes, slowly add 13.45g (0.078 mol) hydrazone, and stirring was continu...
Embodiment 2
[0188] Embodiment 2: preparation tert-butyl 3-isopropyl-3-[(2R,3S)-2-hydroxy-3-(N-quinolinoyl-L-valyl)amino-4-phenylbutyl]carbazate
[0189] Step A: Preparation N-Quinolinoyl-L-valine
[0190] A mixture of 0.62 g (3.6 mmol) of quinolinic acid and 0.61 g (3.76 mmol) of 1,1'-carbonyldiimidazole in 1 ml of dry 1,4-dioxane was stirred for 30 minutes at room temperature. A solution of 0.43 g (3.7 mmol) of L-valine and 0.155 g (3.7 mmol) of lithium hydroxide in 1 ml of water was added thereto, and the resulting mixture was vigorously stirred at room temperature for about 4 hours. The mixture was diluted with water to 10 ml, cooled (ice-water bath), then acidified to pH ca. 3 with 1N hydrochloric acid and allowed to stand at 4°C for 2 hours. The crystals formed were removed by filtration, washed 3 times with 5 ml of cold water and dried over phosphorus pentoxide under high vacuum to give 0.75 g of product. Yield=76%; melting point is 134-136°C;
[0191] NMR (DMSO-d 6 ) 1.03 (...
Embodiment 3
[0196] Embodiment 3: preparation tert-Butyl 3-isopropyl-3-[(2R,3S)-2-hydroxy-3-(N-quinolinoyl-L-aspartoyl)amino-4-phenylbutyl]carbazate
[0197] Step A: Preparation N-quinolinoyl-L-aspartic acid
[0198] When aspartic acid was used instead of L-valine in Step A of Example 2, the title compound was obtained in the same manner, with a melting point of 200-203° C. and a yield of 85%.
[0199] NMR (DMSO-d 6 )3.0 (m, 2H, aSnCH 2 ); 5.0 (m, 1H, aSn CH-2); 6.3 (broad S, 1H, OH); 6.55 (broad 2, 1H, NH 2 ); 7.3 (wide S, 1H, NH 2 ); 7.55-8.6 (m, 6H, aromatic H); 9.22 (d, 1H, NH).
[0200] Step B: Preparation tert-butyl 3-isopropyl-3-[(2R,3s)-2-hydroxy-3-(N-quinolinoyl-L-aspartoyl)amino-4-phenylbutyl]carbazate
[0201] To the product of Step A (0.111g; 0.386mmol), the product of Step B of Example 2 (0.13022g; 0.386mmol), benzotriazol-1-yloxytris(dimethylamino)phosphonium fluorophosphate (0.205g 0.46mmol) and 1-hydroxybenzotriazole (0.052g; 0.384mmol) in 1ml of anhydrous dimet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com