Polycaprolactone with reactive group on side chain and preparation method of polycaprolactone
A technology of polycaprolactone and reactivity, applied in the chemical field, can solve the problems of low hydrophilicity, limited application, lack of active functional groups, etc., and achieve the effect of improving hydrophilicity, simple method, and expanding application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1, azide caprolactone (CL-N 3 )Synthesis
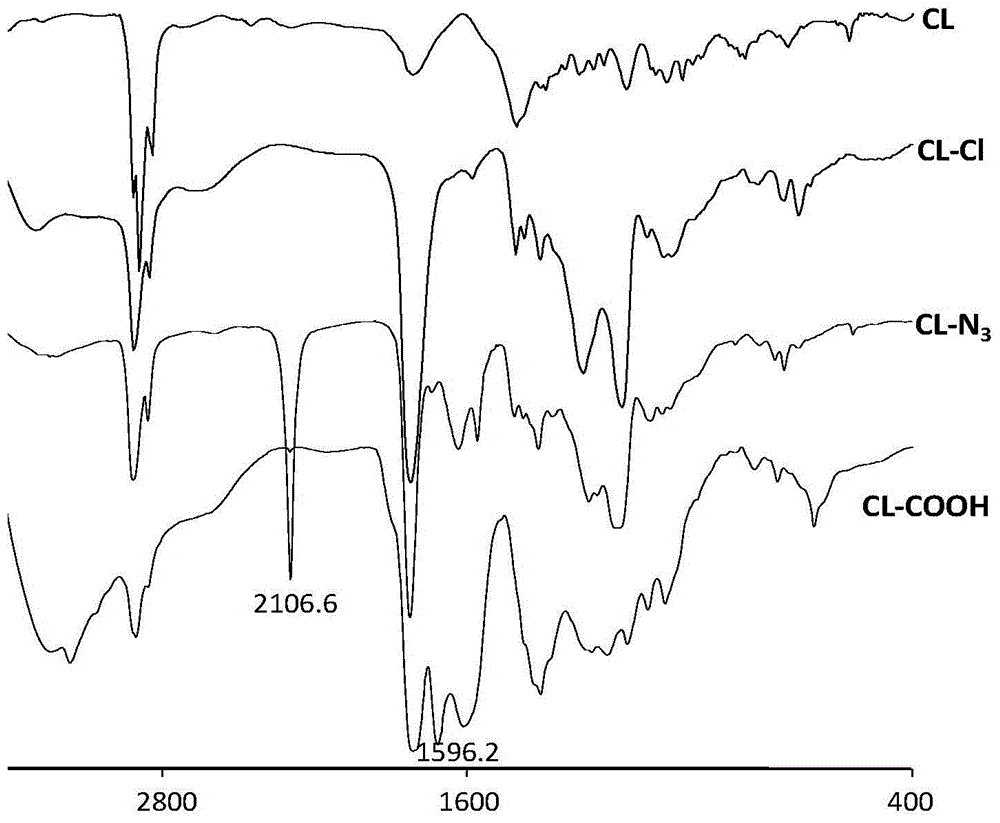
[0031]Dissolve 20 g of α-chlorocaprolactone in 100 mL of dimethylformamide (DMF), stir and dissolve at room temperature, add sodium azide in a molar ratio of 1:1 according to the molar content of chlorine, and stir overnight at 15°C , add diethyl ether to precipitate the product, and wash with diethyl ether to remove DMF, dissolve the obtained product in toluene, centrifuge at 4000r / min for 15min to desalt, take the supernatant after centrifugation, remove toluene under reduced pressure, and obtain 18.2g hexyl azide Esters, denoted as CL-N 3 . CL-N 3 Infrared spectrum detection was carried out, and CL-Cl and ε-CL were used as controls at the same time, the results were as follows figure 1 shown. The results show that at 2106cm -1 appears N 3 The characteristic absorption peaks indicated that the azidation was successful.
Embodiment 2
[0032] Embodiment 2, azide caprolactone (CL-N 3 )Synthesis
[0033] Dissolve 20 g of α-chlorocaprolactone in 100 mL of dimethyl sulfoxide (DMSO), stir and dissolve at room temperature, add sodium azide in a molar ratio of 1:1 according to the molar content of chlorine, and stir overnight at 40°C. Add diethyl ether to precipitate the product, wash with diethyl ether to remove DMSO, dissolve the obtained product in toluene, centrifuge at 4000r / min for 15min to remove salt, take the supernatant after centrifugation, and then distill toluene under reduced pressure to obtain 17.8g hexyl azide Lactone, denoted as CL-N 3 . CL-N 3 Infrared spectrum detection, while using CL-Cl and ε-CL as controls. The obtained infrared spectrum and figure 1 Similar, at 2107cm -1 appears N 3 The characteristic absorption peak indicates that the azidation is successful.
Embodiment 3
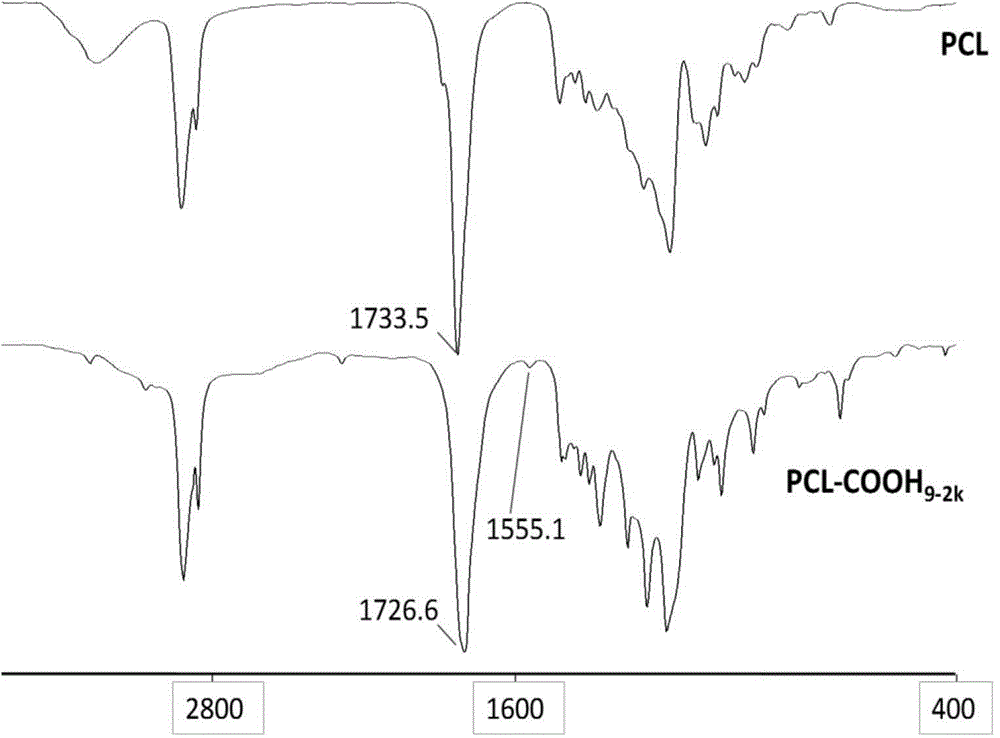
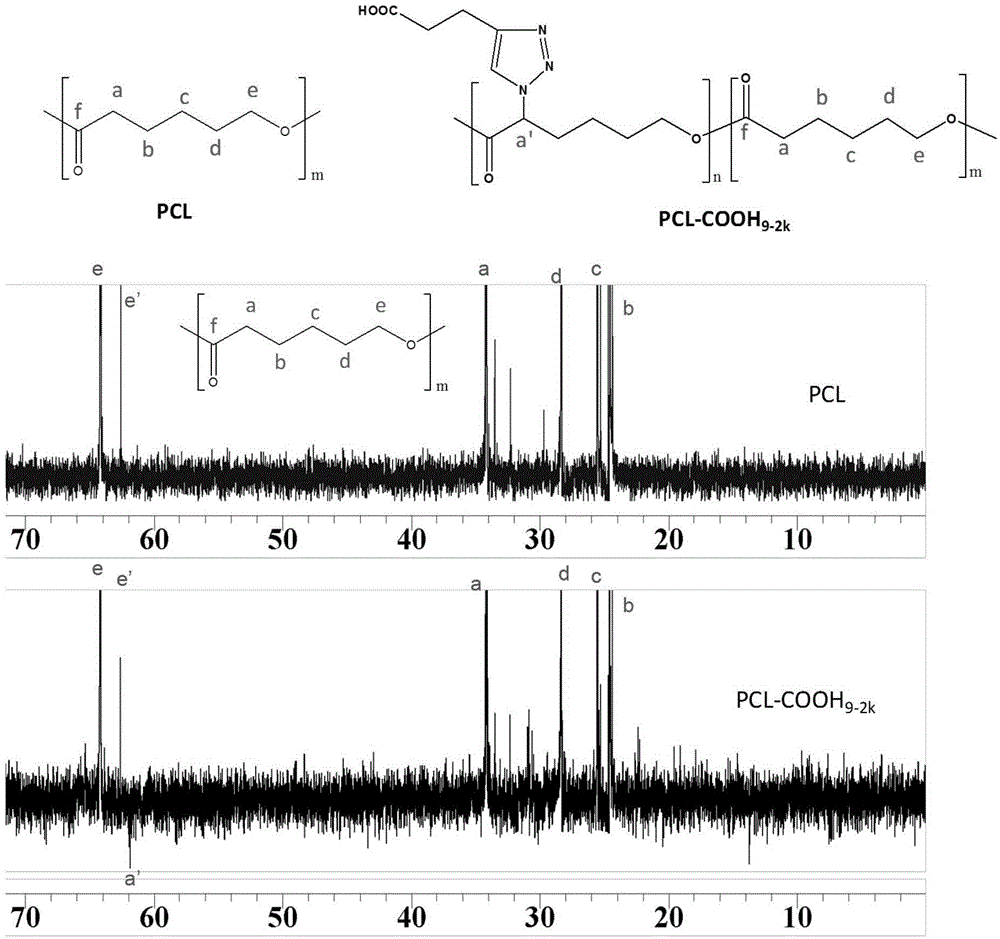
[0034] Embodiment 3, the synthesis of caprolactone (CL-COOH) with-COOH
[0035] Get the CL-N that embodiment 1 makes 3 Dissolve 4.5g in 40mL methanol solvent, add equimolar 4-pentynoic acid, stir to fully dissolve and mix evenly; then add 4mL of 10mg / mL copper sulfate pentahydrate solution; nitrogen exhaust the air in the reaction bottle, and then in Add 6 mL of newly prepared 100 mg / mL sodium ascorbate solution under the protection of nitrogen, stir and react overnight at 25 °C under the protection of nitrogen, distill the methanol off under reduced pressure, dissolve the product in tetrahydrofuran, centrifuge at 4000 r / min for 15 min to remove salt, and take the supernatant after centrifugation Liquid, tetrahydrofuran was distilled off under reduced pressure to obtain 3.5 g of caprolactone with -COOH, denoted as CL-COOH. CL-COOH was detected by infrared spectroscopy, the results are as follows figure 1 shown. The results show that 2106cm -1 Office N 3 The characteristic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water contact angle | aaaaa | aaaaa |
| water contact angle | aaaaa | aaaaa |
| water contact angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com