Solid acid catalyst supporting methane sulfonic acid, preparation method and application of same
The technology of a solid acid catalyst and methanesulfonic acid, which is applied in the polyester synthesis industry, can solve the problems of limited use of liquid acid, waste of national mineral resources, and cumbersome operation process.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Add 21 g of ethyl orthosilicate and 50 g of anhydrous formic acid into the stirring tank, and stir for 30 minutes to obtain a uniform mixed solution. Then, 0.7 g of methanesulfonic acid was added, the stirring intensity was increased, and the stirring was continued for 50 minutes to form a catalyst intermediate. The obtained catalyst intermediate was allowed to stand at 30°C for 6 hours, then transferred to a muffle furnace, and calcined at a temperature of 100°C for 6 hours to obtain a solid acid catalyst.
[0053] The solid acid catalyst samples prepared in this example were evaluated as follows:
[0054] (1)N 2 Adsorption-desorption isotherm,
[0055] (2) Pore size distribution diagram,
[0056] (3) XRD pattern.
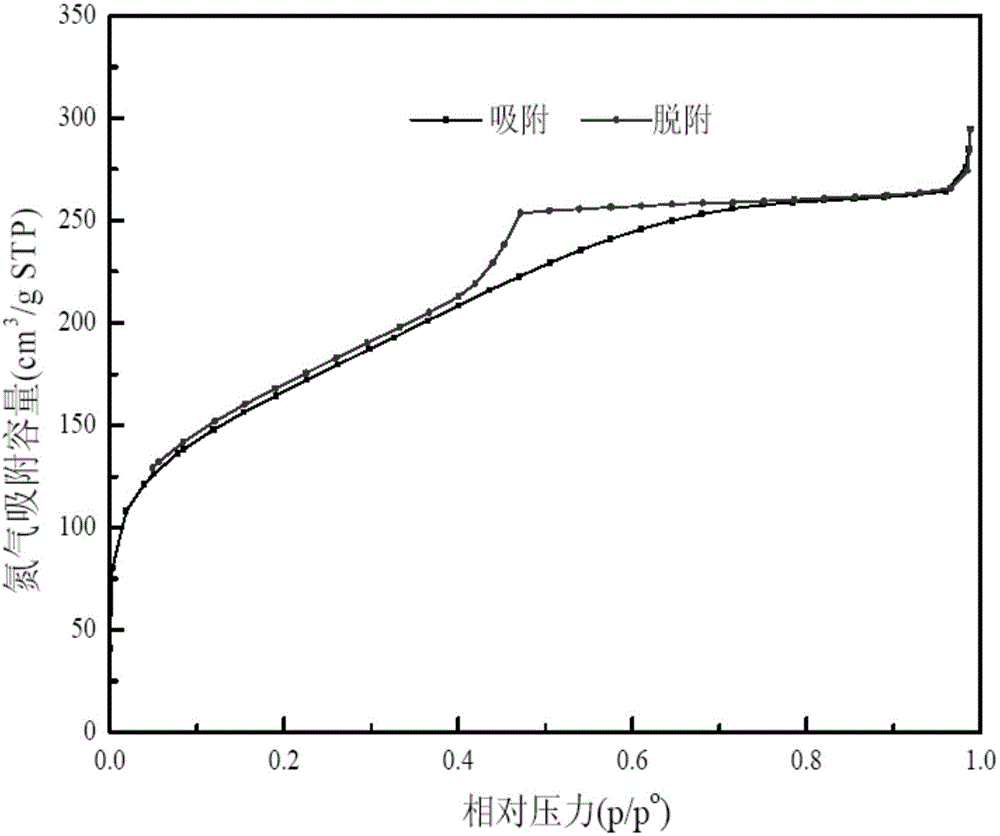
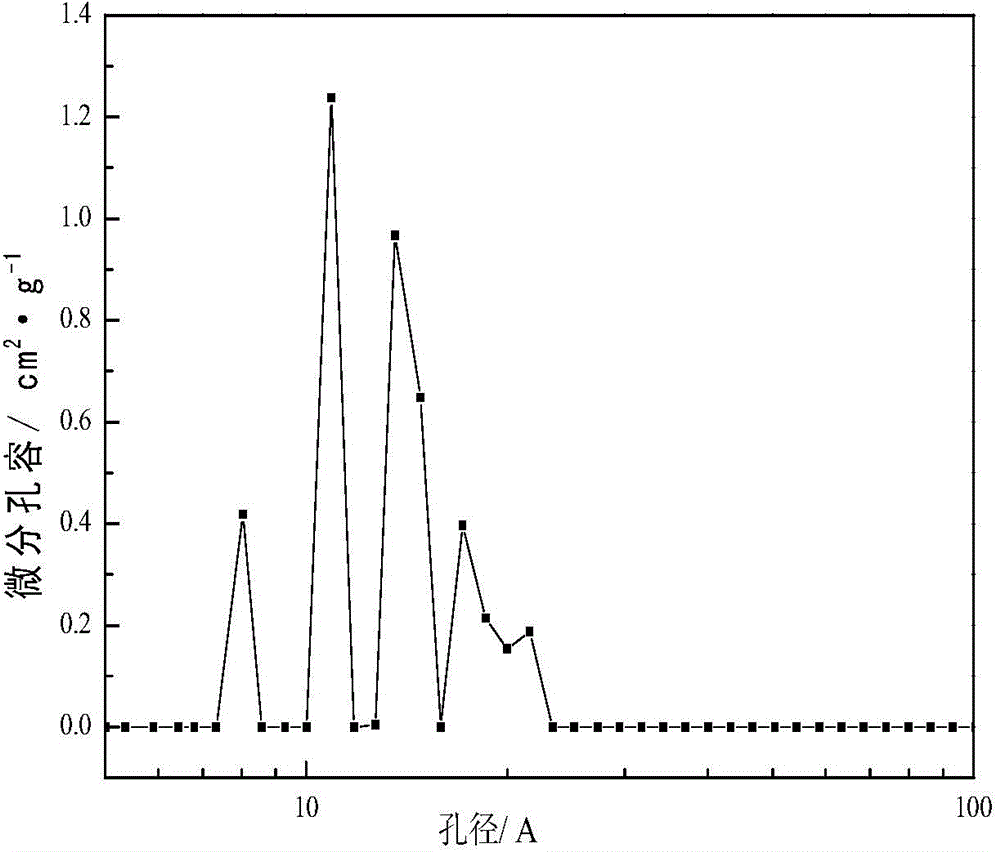
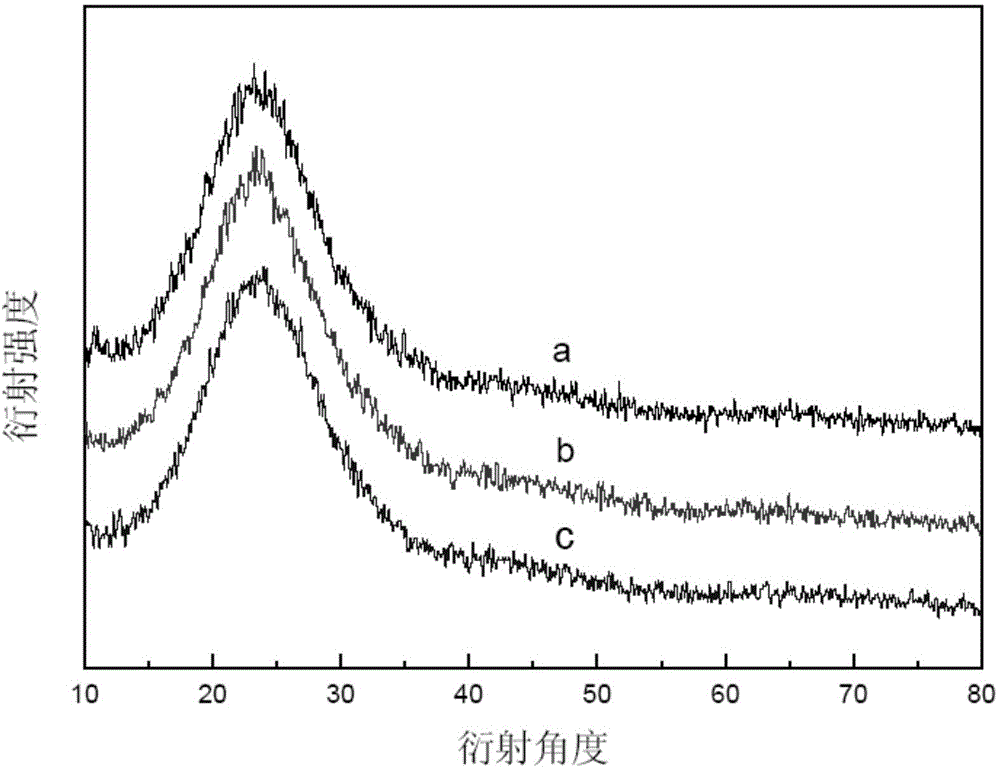
[0057] N of the solid acid catalyst sample obtained in Example 1 2 The adsorption-desorption isotherm curve, pore size distribution diagram, XRD diagram are shown respectively Figure 1-Figure 3 :
[0058] Among them, the N2 adsorption-desorption isotherm shows tha...
Embodiment 2
[0062] Add 21 g of ethyl orthosilicate and 50 g of anhydrous formic acid into the stirring tank, and stir for 30 minutes to obtain a uniform mixed solution. Then, 0.7 g of methanesulfonic acid was added, the stirring intensity was increased, and the stirring was continued for 50 minutes to form a catalyst intermediate. The obtained catalyst intermediate was allowed to stand at 30°C for 6 hours, and then transferred to a muffle furnace and calcined at a temperature of 500°C for 6 hours to obtain a solid acid catalyst.
[0063] As described in Example 1, the solid acid catalyst obtained in Example 2 was evaluated by XRD.
[0064] From image 3 It can be seen from the curves b and c that the solid acid catalyst sample of Example 2 also has the same XRD characteristics of the pure (amorphous) silica support, indicating that the catalyst calcined at 500°C and loaded with methanesulfonic acid is the same as that of Example 1. Compared with the silica structure, the catalyst calcined at ...
Embodiment 3
[0066] Take 2ml of the solid acid catalyst loaded with methanesulfonic acid prepared in Example 1 and Example 2, and put it into a micro fixed bed reactor at a temperature of 100°C, a pressure of 1.0 MPa, and a weight space velocity of 30 h -1 Under the conditions, the reaction is 8 hours. Sampling and analysis are performed every hour, and the bromine index of the sample is analyzed with a bromine index instrument. The catalytic performance curve of the catalyst with the olefin removal rate as a measure is shown in Figure 4 .
[0067] This figure shows that the catalyst still has considerable catalytic performance after being calcined at 500°C, but the catalytic performance is slightly lower than that of the catalyst calcined at 100°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com