Polybenzimidazole with side groups and ether bonds and preparation method and application thereof
A technology of polybenzimidazole and side groups, which is applied in the field of polybenzimidazole and its preparation, can solve the problems of poor solubility, etc., and achieve the effect of increasing solubility, size, oxidation stability and methanol permeability improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
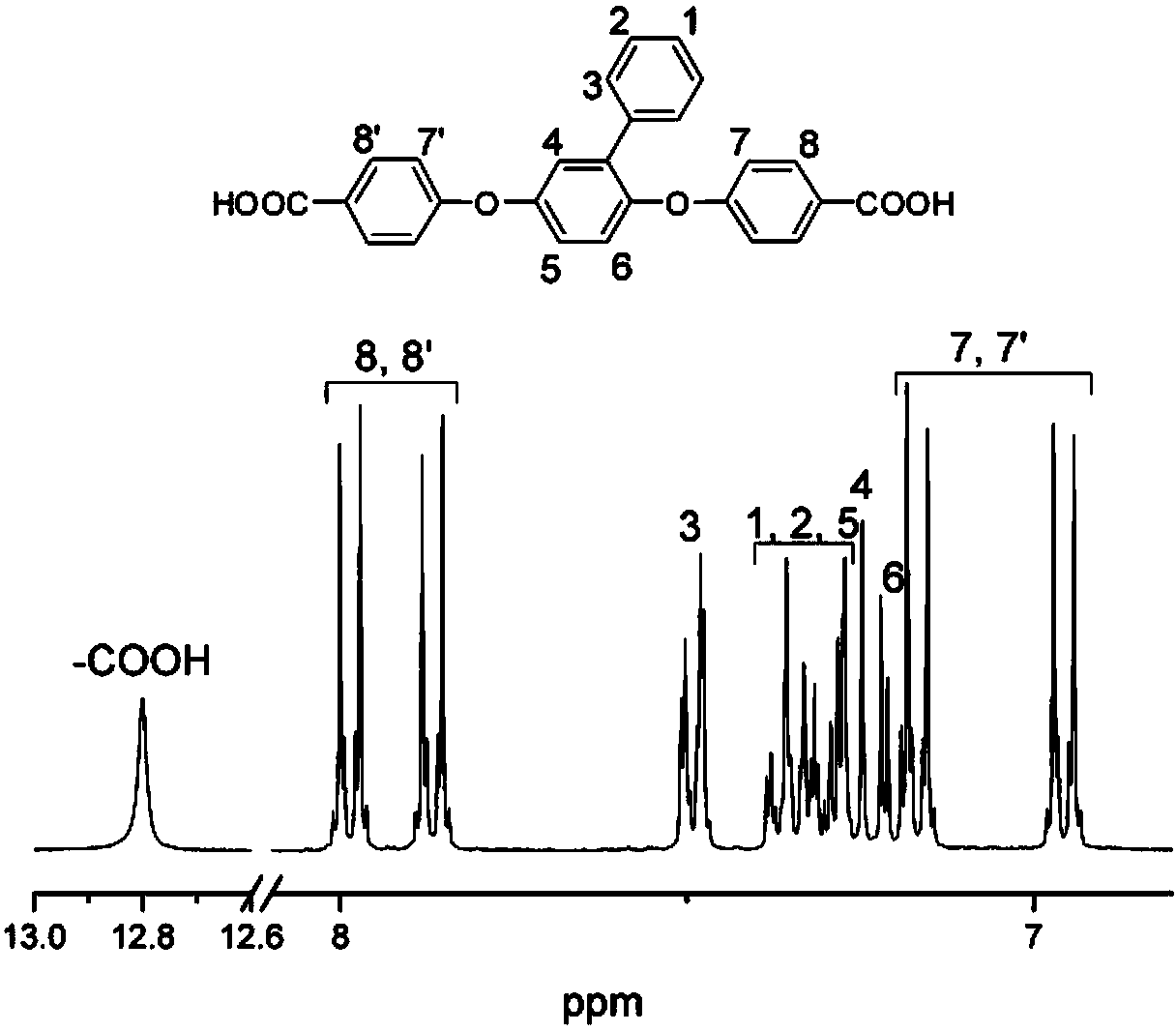
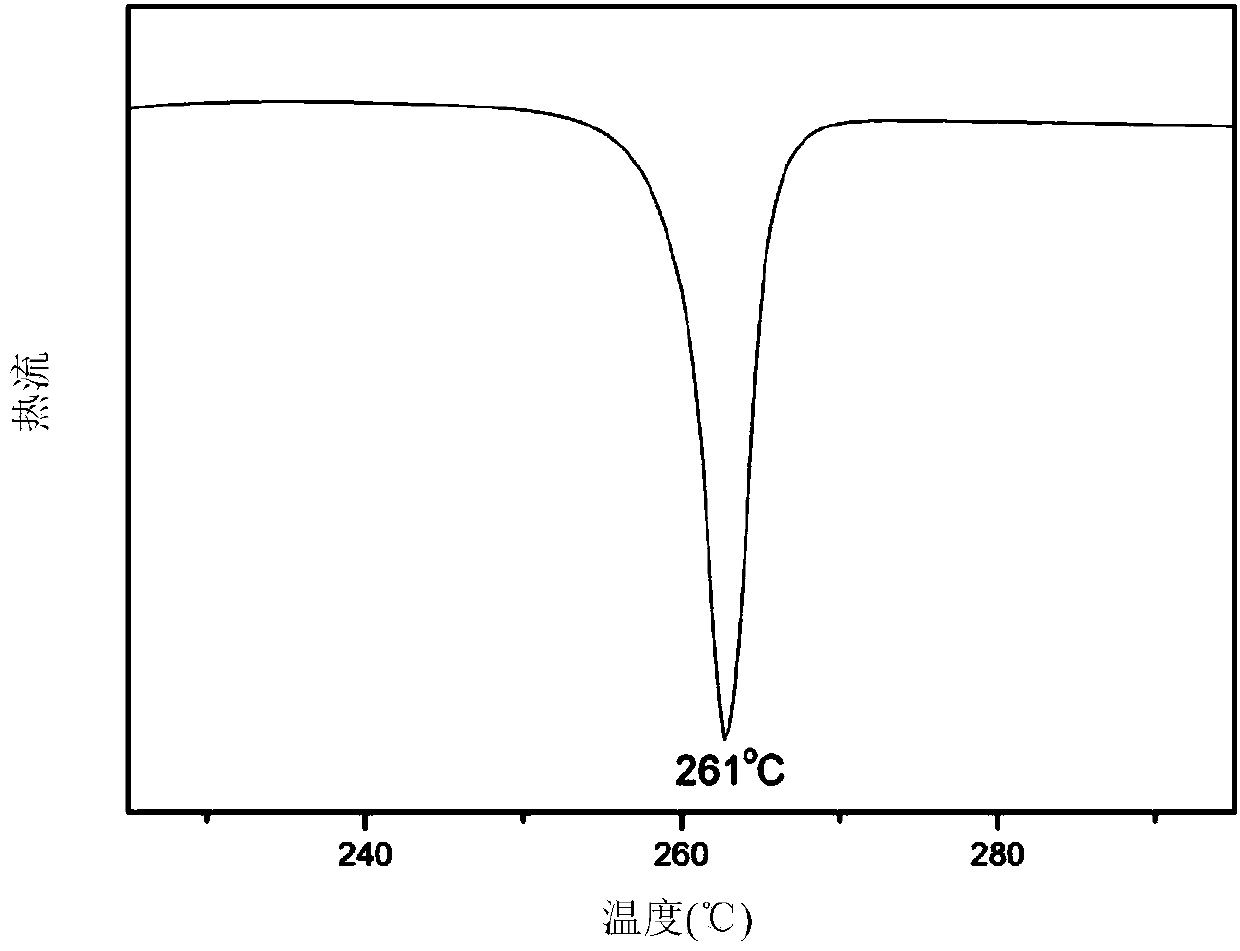
[0043]Dissolve 3.7242g (0.02mol) of 2-phenylhydroquinone in 55mL of a mixed solution of DMF and toluene with a mass ratio of 4:1 (the moles of DMF and toluene are 0.547196 and 0.108530 respectively), and add 5.66579g (0.041 mol) anhydrous potassium carbonate, reflux at 150°C for 3 hours, add 4.96511g (0.041mol) p-fluorobenzonitrile, react at 160°C for 6 hours, and then recrystallize with acetone to obtain diphenylmethane containing benzene side groups and ether bonds Nitrile monomer 6.99156g, its fusing point is 141 ℃, productive rate is 90%;
[0044] Dissolve 6.99156 g (0.018 mol) of the dibenzonitrile monomer containing phenyl side groups and ether bonds in a solution of 140 mL of ethanol and water with a mass ratio of 1:1 (0.017148:0.055556), and add 44.8 g ( 0.8mol) of potassium hydroxide for hydrolysis (the system can be heated to reflux and maintained at reflux for 24 hours). After hydrolysis is complete, the pH of the solution is adjusted to 1 with hydrochloric acid. A...
Embodiment 2
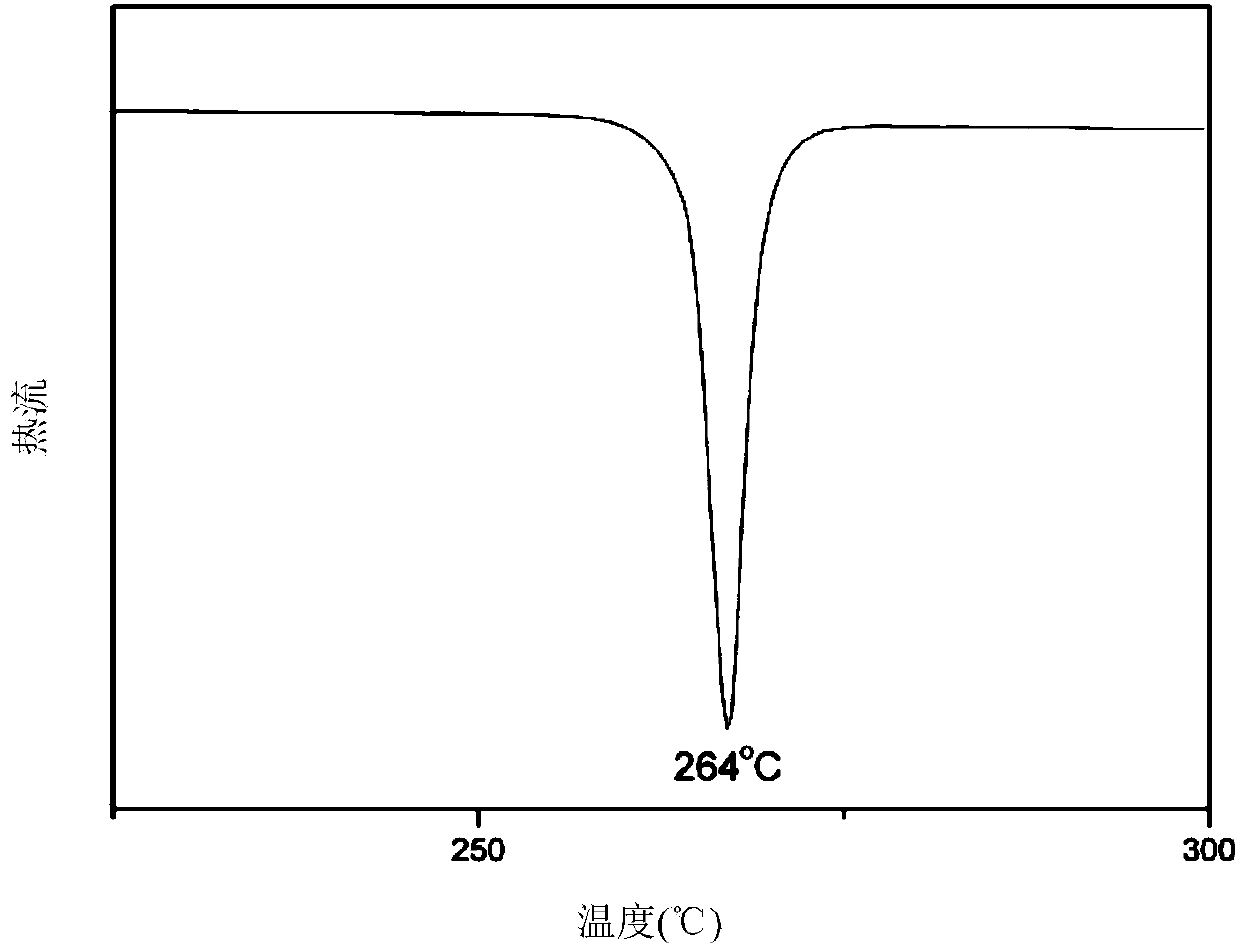
[0049] Using 6.20g (0.045mol) of p-chlorobenzonitrile instead of p-fluorobenzonitrile, and repeating Example 1, 4,4'-(1,4-phenoxy-2-phenyl)dibenzoic acid mono body with a melting point of 264°C and a yield of 81%.
Embodiment 3
[0051] Weigh 5.1654g (0.02mol) of 4-ethyl formate phenylhydroquinone and dissolve it in 55mL of a mixed solution of DMF and toluene with a mass ratio of 4:1 (the moles of DMF and toluene are 0.547196 and 0.108530 respectively), and add 5.66579g (0.041mol) of anhydrous potassium carbonate, reflux at 150°C for 3 hours, add 4.96511g (0.041mol) of p-fluorobenzonitrile, react at 160°C for 6 hours, and recrystallize with acetone to obtain 8.28g of 4,4'-[1,4-phenoxy-2-(3-carboxyethyl)-phenyl]dibenzonitrile monomer, with a melting point of 141°C and a yield of 90%;
[0052] 8.28g (0.018mol) of the 4,4'-[1,4-phenoxy-2-(3-carboxyethyl)-phenyl]dibenzonitrile monomer containing side groups and ether bonds Dissolve in 140mL of ethanol and water with a mass ratio of 1:1 (0.017148:0.055556), and add 22.4g (0.4mol) of potassium hydroxide for hydrolysis (the system can be heated to reflux and maintained at reflux for 24 hours), After the hydrolysis is complete, adjust the pH of the solution t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com