Polyether-ether-ketone bone repair material with multi-scale holes and preparation method thereof
A polyether ether ketone, multi-scale technology, applied in prosthetics, tissue regeneration, medical science, etc., can solve the problems of incomplete removal of pore-forming agents, limited application, and unfavorable bone tissue, etc., to achieve low cost and promote bone tissue. Simple repair and operation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
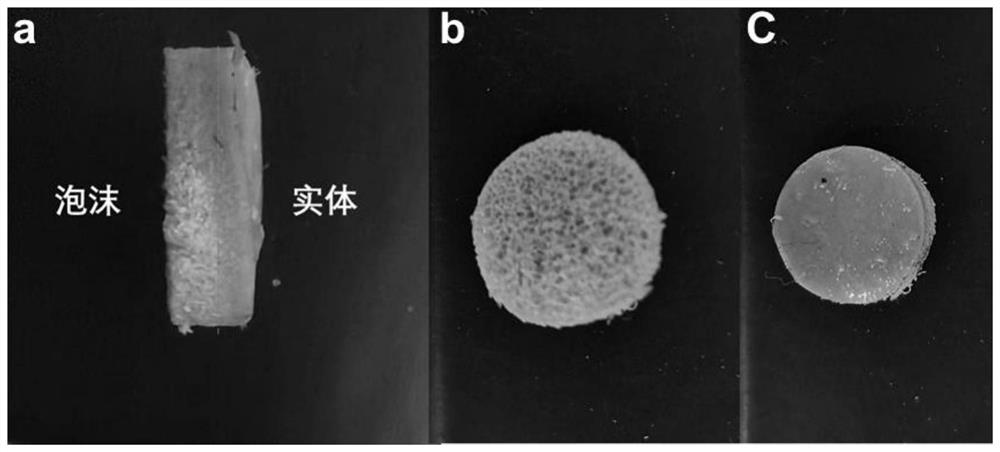
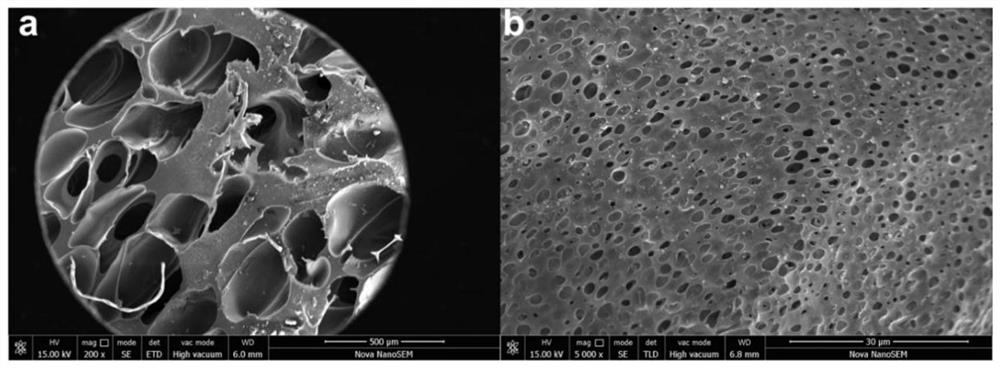
[0023] PEEK pellets (melt index 22g / 10min, purchased from Changchun Jida Special Plastic Engineering Research Co., Ltd.) were placed in a vacuum hot press, and hot-pressed at 375°C and 2MPa to form a polyether ether with a thickness of 2mm. Then cut the polyether ether ketone plate into a disc with a diameter of 14mm, ultrasonically clean it three times with distilled water, ethanol, and acetone, each time for 10 minutes, and then vacuum-dry it at 80°C. The obtained sample is named PEEK. Then place the polyetheretherketone disc in a supercritical carbon dioxide foaming device, foam at 335°C and 10 MPa for 25 minutes, and release the pressure for 25 seconds, and obtain large particles with a size of 200-600 μm on the surface of the polyetheretherketone disc. hole and named it FPEEK. After that, immerse the polyetheretherketone disc and the foamed polyether ether ketone disc in the mixed acid solution for sulfonation (mixing concentrated sulfuric acid with a mass fraction of 98%...
Embodiment 2
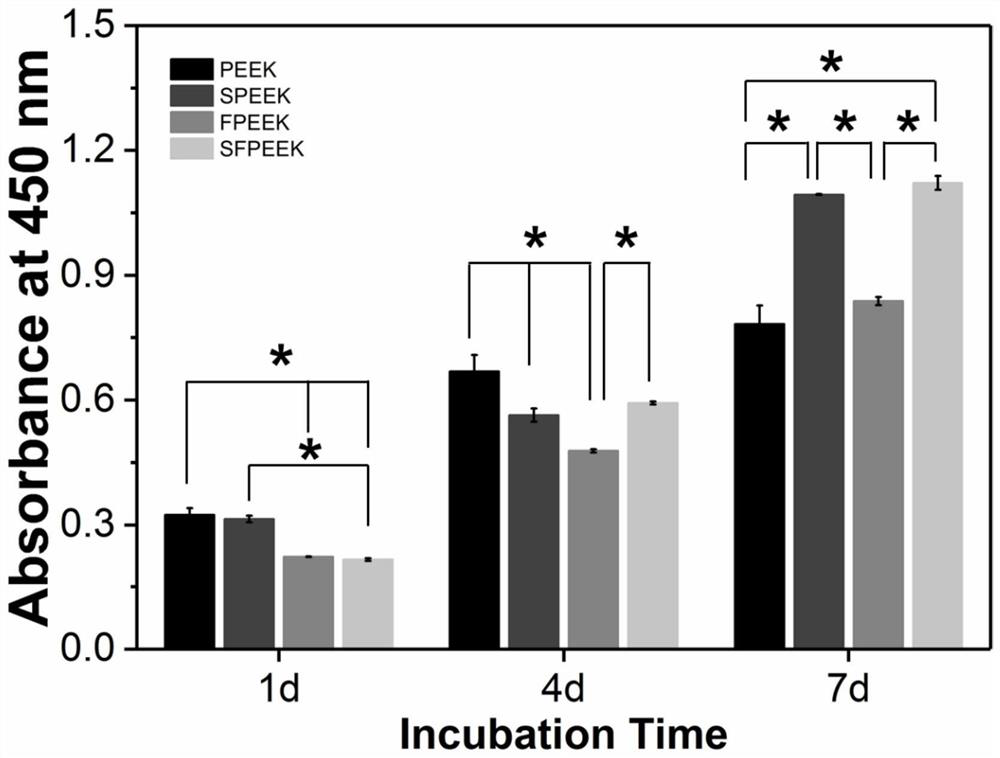
[0026] The samples obtained in Example 1 before and after modification were sterilized in a high-temperature sterilizer. Then placed in a 24-well cell culture plate, the density of each well was 1×10 4 cell / mL of rat bone marrow mesenchymal stem cells (rBMSCs) suspension, then placed in a cell culture incubator at 37°C and 5% carbon dioxide saturated humidity, and the culture medium (containing 10% fetal bovine serum) was changed every 2 days low-sugar DMEM medium). After the cells have been cultured for 1, 4, and 7 days, take out the 24-well culture plate from the cell culture incubator, suck off the old culture medium, and add 200 μL of new culture medium containing 10% CCK-8 (CCK-8, Beyotime, Shanghai, China) Then put it in the cell culture incubator, take it out after 1h, take 100 μL of culture solution from each well and add it dropwise into a 96-well culture plate, use a microplate reader (iMark, Bio-Rad, USA) to measure the concentration of each well at a wavelength of...
Embodiment 3
[0029] The samples obtained in Example 1 before and after modification were sterilized in a high-temperature sterilizer. Then placed in a 24-well cell culture plate, the density of each well was 3×10 4 cell / mL bone marrow mesenchymal stem cells (rBMSCs) suspension, and then placed it in a cell culture incubator at 37°C and 5% carbon dioxide saturated humidity, and changed the culture medium (low glucose containing 10% fetal bovine serum) DMEM medium). After the cells have been cultured for 7 or 14 days, take out the 24-well culture plate from the cell culture incubator, suck off the old culture medium, and wash it with PBS for 3 times, 15 minutes each time, and then lyse it with 0.5% Triton-100 for 1 hour, and collect the lysed cells liquid. Then, the alkaline phosphatase (ALP) activity was obtained according to the instructions of the alkaline phosphatase kit (NanjingJiancheng, Nanjing, China) and the BCA protein concentration assay kit (BCA Protein Assay Kit, Shanghai, Chi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melt flow index | aaaaa | aaaaa |
| melt flow index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com