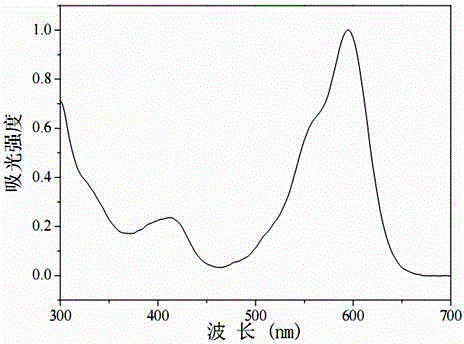
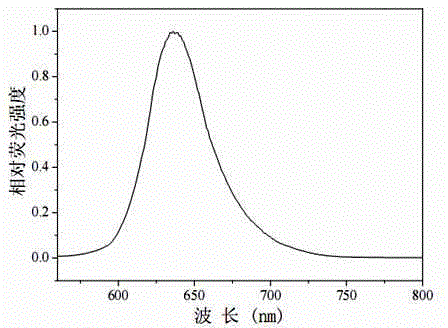
Asymmetric near-infrared BODIPY fluorescent dye as well as preparation method and application thereof
A fluorescent dye and near-infrared technology, which is used in the preparation and application of fluorescent dyes, can solve the problems of poor water solubility, limited application, and lack of groups, and achieve the effects of saving raw materials, simple and easy preparation methods, and good photostability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
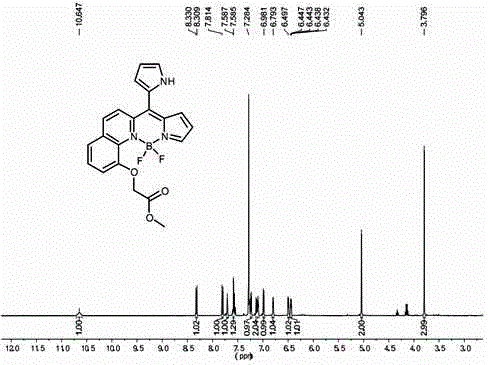
[0033] A kind of asymmetric near-infrared BODIPY fluorescent dye, chemical formula is C 18+m h 13+n BF 2 N 2+x o y , where m is 3, n is 4, x is 1, y is 3, and the molecule has the following structure:
[0034] ,
[0035] where: R 1 , R 2 , R3 for H, R 4 is acetoxy, R 5 , R 6 is a fluorine atom,
[0036] Its preparation method steps are as follows:
[0037] 1) Dissolve 0.34 ml (2.4 mmol) of pyrrole in 50 ml of toluene, add 220 mg (1 mmol) of 8-acetoxyquinoline-2-carbaldehyde under nitrogen atmosphere to obtain a mixed solution, 2) under nitrogen protection , adding 5 microliters of catalyst trifluoroacetic acid dropwise to the reaction solution, and reacting at a temperature of 0° C. for 3 hours to obtain a reaction solution;
[0038] 3) Add 245 mg of 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) to the above reaction solution, the mixture of DDQ and 8-acetoxyquinoline-2-carbaldehyde The molar ratio is 1.1:2, and the oxidative dehydrogenation is carried out for...
Embodiment 2
[0046] Embodiment 2: A kind of asymmetrical near-infrared BODIPY fluorescent dye, chemical formula is C 18+m h 13+n BF 2 N 2+x o y , where m is 3, n is 4, x is 1, y is 3, and the molecule has the following structure:
[0047] ,
[0048] where: R 1 , R 2 , R 3 for H, R 4 is acetoxy, R 5 , R 6 is a fluorine atom,
[0049] Its preparation method steps are as follows:
[0050] 1) 7.0 ml of pyrrole was dissolved in 10 ml of dichloromethane, and 0.10 ml of 8-acetoxyquinoline-2-carbaldehyde was added under a nitrogen atmosphere to obtain a mixed solution;
[0051] 2) Under nitrogen protection, 5 microliters of the catalyst p-toluenesulfonic acid was added dropwise to the reaction liquid, and reacted at a temperature of 100°C for 3 hours to obtain a reaction liquid;
[0052] 3) Add 2.24 grams of 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) to the above reaction solution, the mixture of DDQ and 8-acetoxyquinoline-2-carbaldehyde The molar ratio is 10.0:1, and the oxid...
Embodiment 3
[0059] A kind of asymmetric near-infrared BODIPY fluorescent dye, chemical formula is C 18+m h 13+n BF 2 N 2+x o y , where m is 3, n is 4, x is 1, y is 3, and the molecule has the following structure:
[0060] ,
[0061] where: R 1 , R 2 , R 3 for H, R 4 is acetoxy, R 5 , R 6 is a fluorine atom,
[0062] Its preparation method steps are as follows:
[0063] 1) Dissolve 0.2 ml of pyrrole in 50 ml of ether, and add 0.10 ml of 8-acetoxyquinoline-2-carbaldehyde under nitrogen atmosphere to obtain a mixed solution;
[0064] 2) Under the protection of nitrogen, add 5 microliters of catalyst trifluoroacetic acid dropwise to the reaction solution, and react at a temperature of 20°C for 3 hours to obtain a reaction solution;
[0065]3) Add 2.3 mg of 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) to the above reaction solution, the mixture of DDQ and 8-acetoxyquinoline-2-carbaldehyde The molar ratio is 0.01:1, and the oxidative dehydrogenation is carried out for 0.5 ho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com