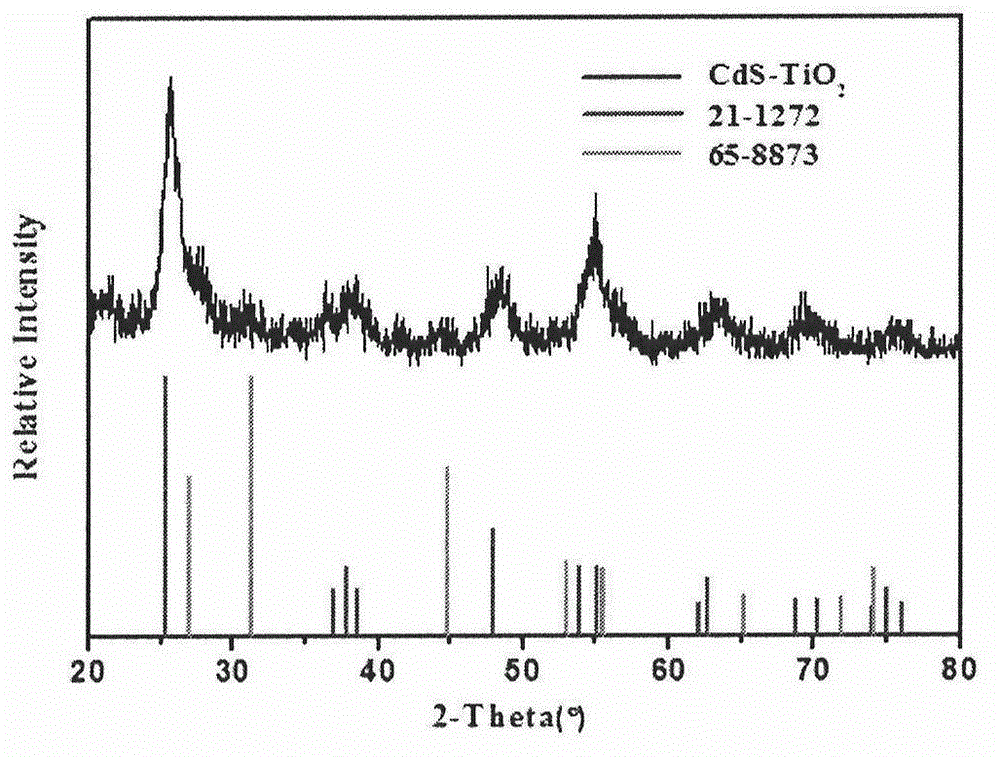
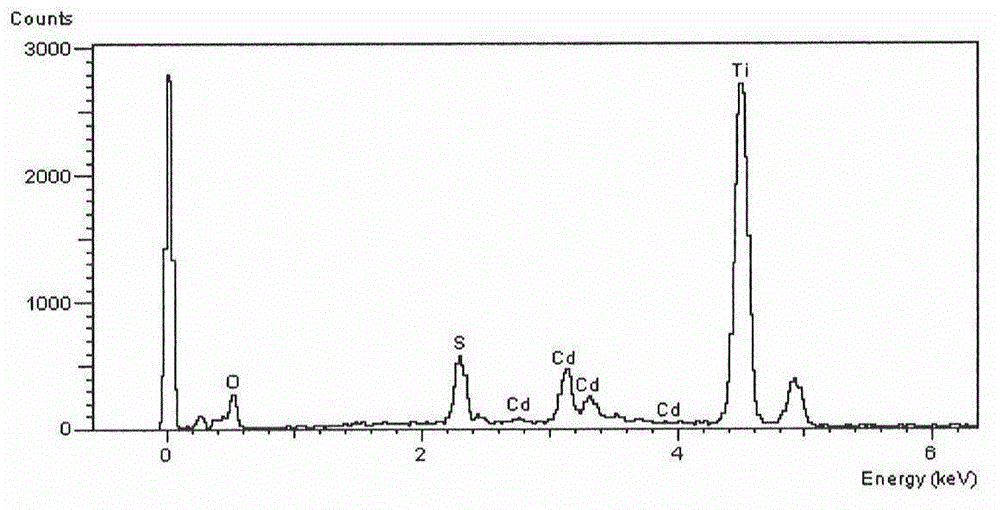
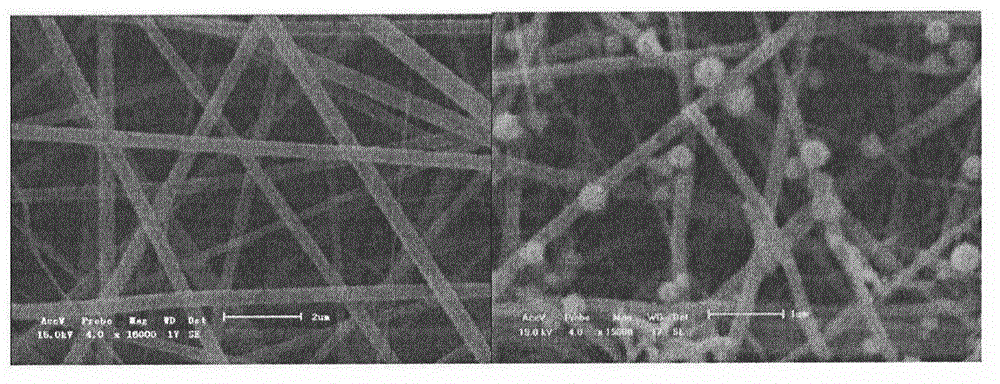
Catalyst for catalytic degradation of pollutants by ultraviolet light and preparation method of catalyst
A catalytic degradation and catalyst technology, which is applied in the field of pollutant treatment, can solve the problems of harsh reaction conditions, high cost, and long reaction time, and achieve the effects of mild reaction conditions, easy operation, and improved efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: 10g tetrabutyl titanate (TBT), 0.089g cadmium acetate dihydrate (Cd(Ac) 2 2H 2 O), 7mL acetic acid, 120mL methanol and 5.6g polyvinylpyrrolidone (PVP) were poured into a 250mL Erlenmeyer flask. After stirring at room temperature for 4-8h, PVP / Cd(Ac) was obtained 2 - TBT sol precursor. After electrospinning the above precursor, calcined at 500°C for 4h, cooled at room temperature, uniformly dispersed in the aqueous solution of 0.05g thioacetamide dissolved, after hydrothermal reaction at 70°C for 12h, washed with water and ethanol three times respectively, centrifuged and dried in vacuum , making 1% CdS-TiO 2 catalyst. Take 100mg catalyst and disperse it in 1L 20mg / L -1 In the methyl orange solution, after dark adsorption for 30min, UV light was applied, and 5mL supernatant was taken out at regular intervals to test its absorbance, and the reaction was terminated after 5h.
Embodiment 2
[0023] Embodiment 2: 10g tetrabutyl titanate (TBT), 0.267g cadmium acetate dihydrate (Cd(Ac) 2 2H 2 O), 7mL acetic acid, 120mL methanol and 5.6g polyvinylpyrrolidone (PVP) were poured into a 250mL Erlenmeyer flask. After stirring at room temperature for 4-8h, PVP / Cd(Ac) was obtained 2 - TBT sol precursor. After electrospinning the above precursor, calcined at 500°C for 4h, cooled at room temperature, uniformly dispersed in the aqueous solution of 0.15g of thioacetamide dissolved, after hydrothermal reaction at 70°C for 12h, washed with water and ethanol three times respectively, centrifuged and dried in vacuum , made 3%CdS-TiO 2 catalyst. Take 100mg catalyst and disperse it in 1L 20mg / L -1 In the methyl orange solution, after dark adsorption for 30min, UV light was applied, and 5mL supernatant was taken out at regular intervals to test its absorbance, and the reaction was terminated after 5h.
Embodiment 3
[0024] Embodiment 3: 10g tetrabutyl titanate (TBT), 0.445g cadmium acetate dihydrate (Cd(Ac) 2 2H 2 O), 7mL acetic acid, 120mL methanol and 5.6g polyvinylpyrrolidone (PVP) were poured into a 250mL Erlenmeyer flask. After stirring at room temperature for 4-8h, PVP / Cd(Ac) was obtained 2 - TBT sol precursor. After electrospinning the above precursor, calcined at 500°C for 4h, cooled at room temperature, uniformly dispersed in an aqueous solution of 0.25g of thioacetamide dissolved, reacted hydrothermally at 70°C for 12h, washed with water and ethanol three times, centrifuged, and dried in vacuum , making 5% CdS-TiO 2 catalyst. Take 100mg catalyst and disperse it in 1L 20mg / L -1 In the methyl orange solution, after dark adsorption for 30min, UV light was applied, and 5mL supernatant was taken out at regular intervals to test its absorbance, and the reaction was terminated after 5h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com