Organic luminescent material and application thereof
A luminescent material and organic technology, applied in luminescent materials, organic chemistry, electrical components, etc., can solve problems such as easy breakage, and achieve the effects of increasing service life, improving luminous efficiency, and good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] For the preparation embodiment of the intermediate of the present invention:
[0056] Synthesis of Main Intermediate Naphthalene-2,5-Diboronic Acid
[0057]
[0058] Dissolve 5.7g of 2,6-dibromonaphthalene (molecular weight 284, 0.02mol) in 100ml of dry THF, add 20ml of n-butyl (2.5M, 0.05mol) dropwise at -80°C, stir for 15min, then add dropwise Triisopropyl borate 30ml. Hydrolyze, adjust the pH to neutral, and precipitate 4.35 g of white boric acid derivatives, with a yield of nearly 100%.
Embodiment 2
[0059] Synthesis of compound shown in embodiment 2 formula (5)
[0060] Step S1,
[0061]
[0062] 1000 ml one-necked bottle, equipped with a magnetic stirrer, add 4.35 g of naphthalene-2,6 diboronic acid (molecular weight 216, 0.02 mol), 11.4 g of 2,4-dibromonitrobenzene (molecular weight 278, 0.041 mol), Pd (PPh 3 ) 4 Use amount 2.6g (molecular weight 1154, 0.00253mol), sodium carbonate 150ml (2M), toluene 150ml, ethanol 150ml. After argon replacement, reflux, and monitor the reaction with TLC, the reaction was complete after 3 hours, lowered the temperature, separated the base layer, evaporated to dryness, and carried out column separation with ethyl acetate / petroleum ether to obtain 9.75g product, molecular weight 526, yield 92.5 %.
[0063] Step S2,
[0064]
[0065] In a 50 ml bottle equipped with a magnetic stirrer, add 9.75 g of the final product of the first step (molecular weight 526, 0.0185 mol), 10.4 g of triphenylphosphine (molecular weight 262, 0.0395 m...
Embodiment 3
[0073] Synthesis of compound shown in embodiment 3 formula (6)
[0074] The synthesis steps are the same as the four-step reaction in Example 2, except that in step S3, iodobenzene is changed to 2-iodonaphthalene to obtain compound 2.
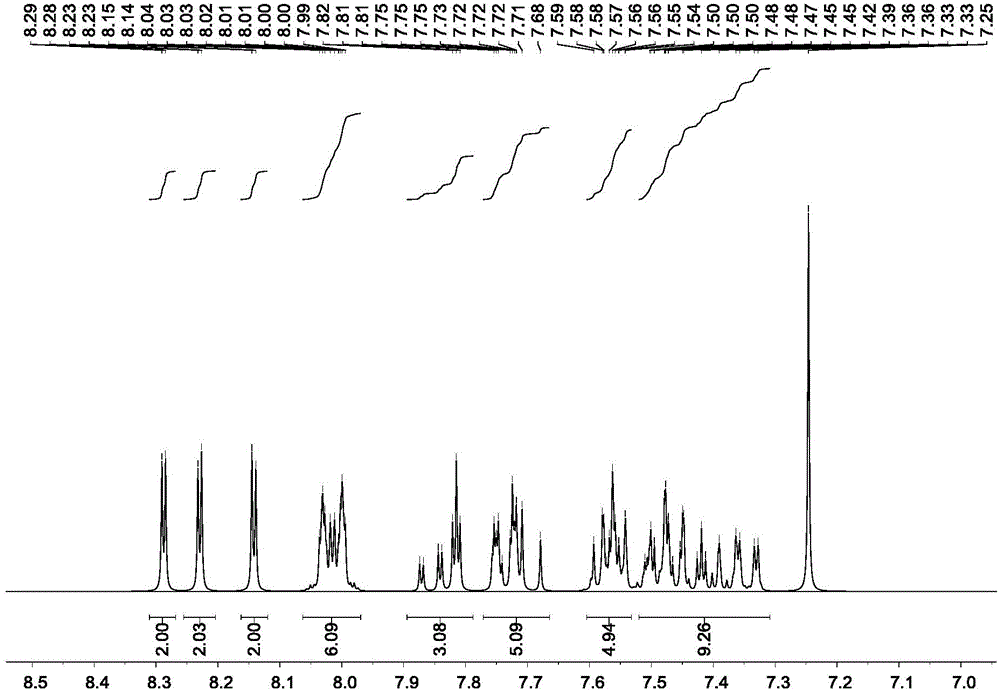
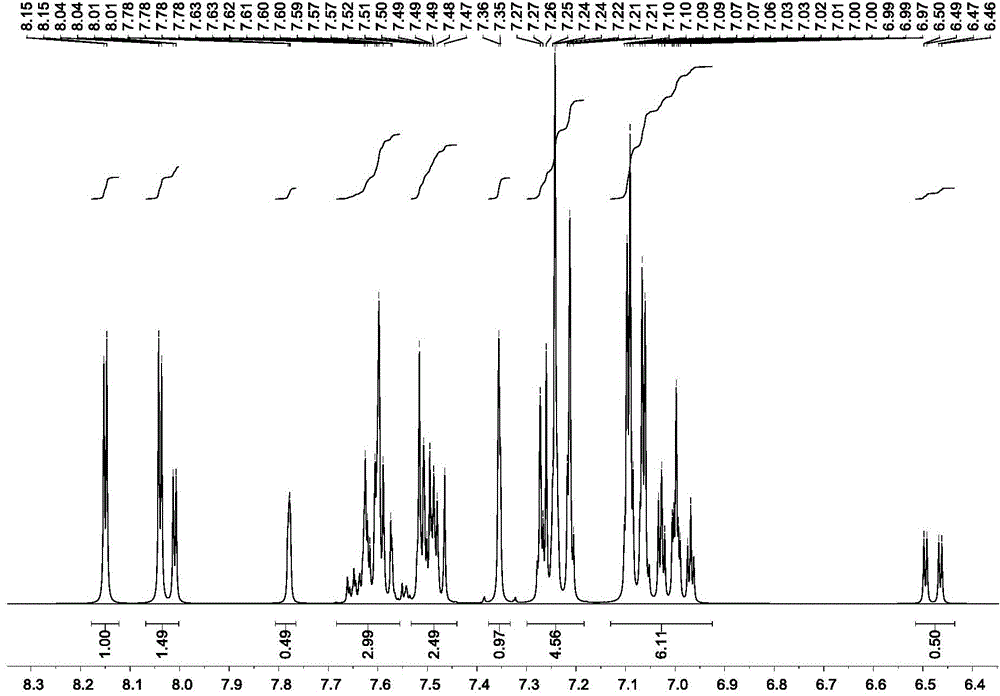
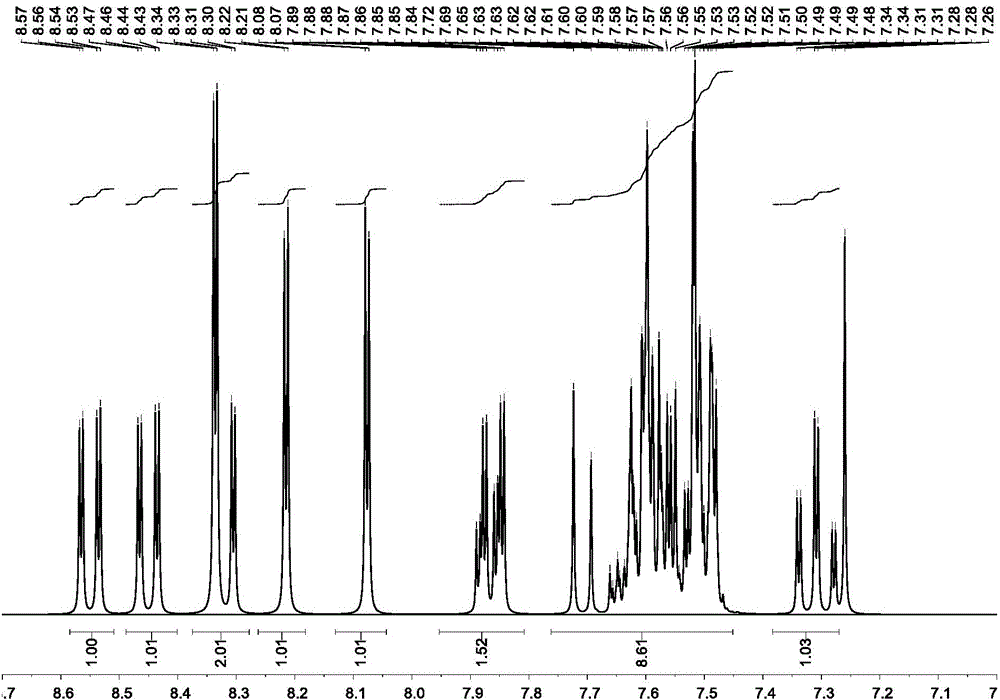
[0075] Product MS (m / e): 710, elemental analysis (C 54 h 34 N 2 ): theoretical value C: 91.24%, H: 4.82%, N: 3.94%; measured value C: 91.27%, H: 4.80%, N: 3.93%, the nuclear magnetic spectrum of the compound ( 1 HNMR) see attached figure 1 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com