Cadmium metal complex with dye catalytic light degradation property and preparation method of cadmium metal complex
A complex and photodegradation technology, applied in the chemical field, can solve the problems of wide energy gap, low utilization rate of visible light, low quantum efficiency, etc., and achieve the effects of high yield, stable photocatalytic degradation dye performance, and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0012] The preparation method of the cadmium metal complex having the property of catalytic photodegradation dyestuff comprises the following steps: dihydrate cadmium acetate, 5-methylisophthalic acid, 1,3-bis(pyridine-3-methoxy)benzene and distilled water into a hard glass tube and seal the tube, then put the glass tube in an oven and react at 170°C for four days. After the reaction is completed, the glass tube is slowly cooled at a rate of 5°C / h. To obtain a cadmium coordination polymer, collect colorless massive crystals and let them dry naturally in the air. Further, the molar ratio of the raw materials used is: cadmium acetate dihydrate: 5-methylisophthalic acid: 1, 3-bis(pyridine-3-methoxy)benzene=2:1:1, further, the number of liters of distilled water used is 100 times the mole number of cadmium acetate dihydrate.
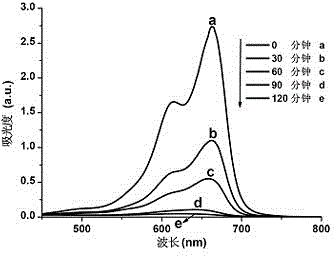
[0013] A cadmium metal complex with the property of catalyzing photodegradation of dyes, and the complexes are used for photodegradation of dyes.
Embodiment 1
[0014] Embodiment 1: the synthesis of complex:
[0015] Cadmium acetate dihydrate (11 mg, 0.04 mmol), 5-methylisophthalic acid (4 mg, 0.02 mmol), 1,3-bis(pyridine-3-methoxy)benzene (6 mg, 0.02 mmol ) and 4mL of distilled water were put into a hard glass tube and sealed, then the glass tube was put into an oven and reacted at 170°C for four days. Finally, the colorless blocky crystals were collected and dried naturally in the air to obtain the cadmium coordination polymer. The yield is about 43%. The reproducibility of this method is good after repeated tests.
Embodiment 2
[0016] Embodiment 2: the characterization of complex:
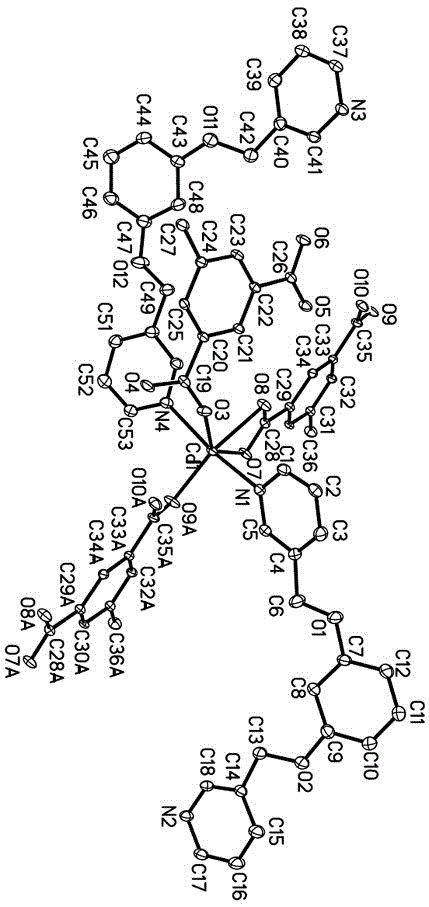
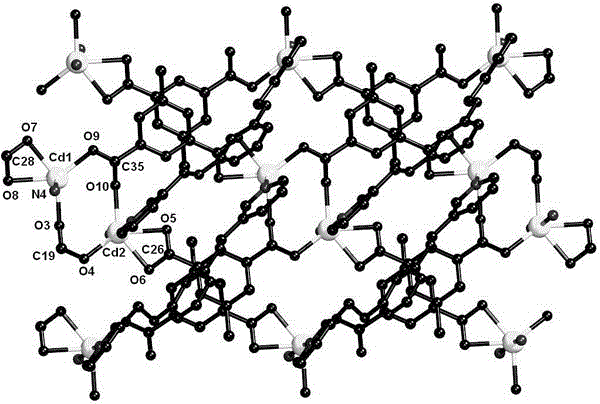
[0017]Determination of the structure of the complex: select a single crystal of an appropriate size under a microscope to conduct X-ray diffraction experiments at room temperature. On a Bruker Smart Apex-II CCD diffractometer, Mo–K α Rays (wavelength = 0.71073 ?), collect diffraction data in the φ–ω manner. Data restoration was performed with the Bruker SAINT program. Diffraction data for partial structures were corrected for absorption using the SADABS program. The crystal structure was solved by direct method combined with difference Fourier synthesis. The coordinates and anisotropy parameters of all non-hydrogen atoms were corrected by the full matrix least squares method, and the positions of C–H atoms were calculated and determined according to the theoretical model. The detailed crystal measurement data are shown in Table 1. For the crystal structure of the complex, see figure 2 . Mo–K α The subscript of K i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com