Nimodipine fat emulsion concentrate and preparation method and use thereof
A technology for nimodipine fat and emulsion concentrate, which is applied in the fields of emulsion delivery, cardiovascular system diseases, nervous system diseases, etc., and can solve the problems of poor doctor-patient compliance, irritation, and poor water solubility of nimodipine.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
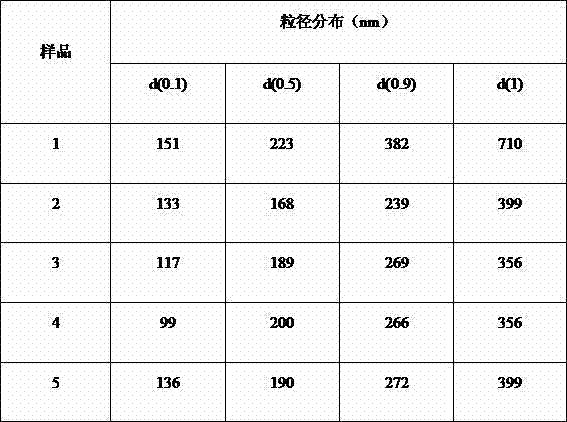
[0139] Embodiment 1: the preparation of nimodipine fat emulsion concentrate sample 1
[0140] Its composition is as follows:
[0141] Nimodipine 0.25g
[0142] Medium-chain fatty acid glycerides (Crodamol, GTCC) 29 grams
[0143] soybean oil 29 grams
[0144] 1, 2-propanediol 8.7g
[0145] Soy Lecithin 10g
[0146] Polyglyceryl Oleate 3.3g
[0147] Polyethylene glycol lauryl hydroxystearate (Solutol HS 15, BASF) 10.6 g
[0148] Tween 80 8.7g
[0149] Oleic acid 0.25g
[0150] Vitamin E 0.2g
[0151] The nimodipine fat emulsion concentrate preparation method is as follows:
[0152] 1) At 20°C, add the above-mentioned weight of soybean lecithin and polyglycerol oleate into medium-chain fatty acid glycerides and soybean oil, and stir at 20,000 rpm until a transparent and clear solution is formed;
[0153] 2) Add the above weight of nimodipine to the product of step 1) at 20°C, and keep stirring at 200rpm until the whole system is transparent;
[0154] 3) Add the above-...
Embodiment 2
[0156] Embodiment 2: the preparation of nimodipine fat emulsion concentrate sample 2
[0157] Its composition is as follows:
[0158] Nimodipine 0.5g
[0159] Medium-chain fatty acid glycerides (Miglyol 812, SASOL) 67g
[0160] 1, 2-Propanediol 8.3g
[0161] Soy Lecithin 7.5g
[0162] Polyglyceryl Oleate 4.2g
[0163] Polyethylene glycol lauryl hydroxystearate (Solutol HS 15, BASF) 8.3 g
[0164] Tween 80 4g
[0165] Oleic acid 0.2g
[0166] The nimodipine fat emulsion concentrate preparation method is as follows:
[0167] 1) At 45°C, add the above-mentioned weight of soybean lecithin and polyglycerol oleate to medium-chain fatty acid glycerides, and stir at 2000rpm until a transparent and clear solution is formed;
[0168] 2) Add the above-mentioned weight of nimodipine to the product of step 1) at 35°C, and keep stirring at 1000rpm until the whole system is transparent;
[0169] 3) Add the above-mentioned weight of 1,2-propanediol, Tween 80, polyethylene glycol laur...
Embodiment 3
[0171] Embodiment 3: the preparation of nimodipine fat emulsion concentrate sample 3
[0172] Its composition is as follows:
[0173] Nimodipine 0.5g
[0174] Medium-chain fatty acid glycerides (Miglyol 812, SASOL) 67g
[0175] 1,2-propanediol 9 grams
[0176] Soy Lecithin 12g
[0177] Polyethylene glycol lauryl hydroxystearate (Solutol HS 15, BASF) 7.5 g
[0178] Tween 80 3.5g
[0179] Oleic acid 0.3g
[0180] Vitamin E 0.2g
[0181] The nimodipine fat emulsion concentrate preparation method is as follows:
[0182] 1) At 30°C, add the above-mentioned weight of soybean lecithin to medium-chain fatty acid glycerides, and stir at 10,000 rpm until a transparent and clear solution is formed;
[0183] 2) Add the above weight of nimodipine to the product of step 1) at 45°C, and keep stirring at 2000rpm until the whole system is transparent;
[0184] 3) Add the above-mentioned weight of 1,2-propanediol, polyethylene glycol lauryl hydroxystearate, Tween 80, oleic acid, and vi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com