Preparation method of catalyst for alkyne selective hydrogenation
A technology for selective hydrogenation and catalysts, applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, etc. poor selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
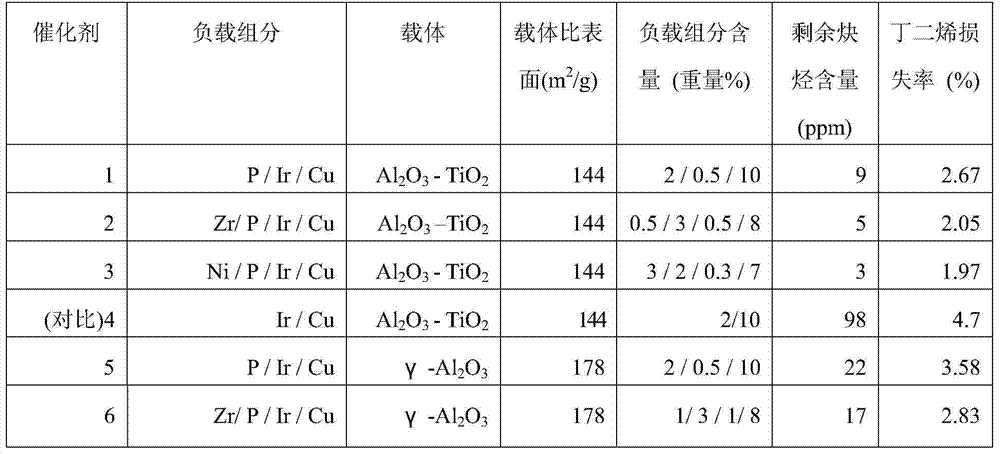
[0048] Embodiment 1—preparation of catalyst 1
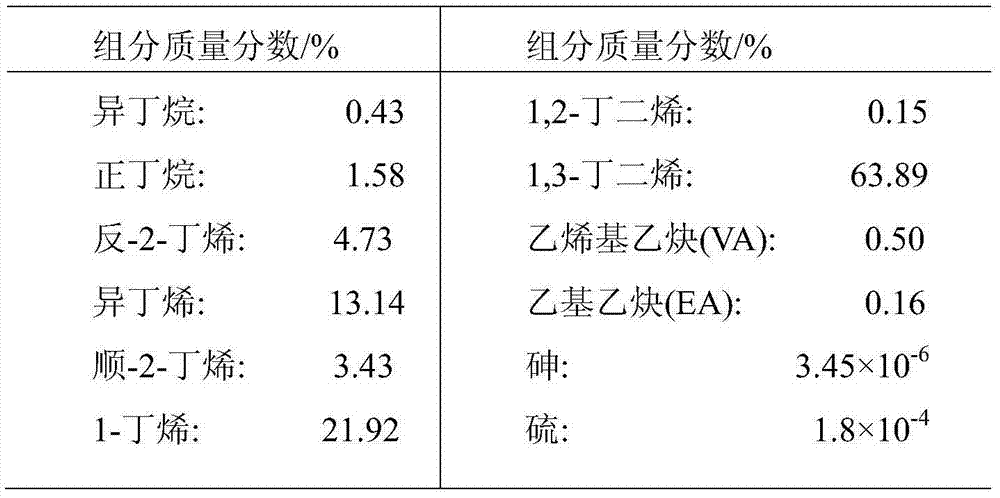
[0049] Firstly, a titania-alumina composite oxide carrier was prepared according to the method in Example 1 of CN1184289C. Take the specific surface area as 160m 2 / g, 90g of clover-shaped alumina with a pore volume of 0.58mL / g and a most probable pore diameter of 130 angstroms, impregnated with 0.557g / mL dilute sulfuric acid solution of 63 ml of titanium sulfate, stirred for 15 minutes, and dried at 120°C for 8 hours Then, it was calcined at 900° C. for 4 hours to obtain a titanium oxide-alumina composite oxide (A-1). The obtained composite oxide had a titanium oxide content of 10% by weight and a specific surface area of 144m 2 / g, the pore volume is 0.56mL / g, and the most probable pore diameter is 125 angstroms.
[0050] Prepare 63mL of a mixed aqueous solution containing copper nitrate, ammonium phosphate, and iridium trichloride as the immersion solution. In the immersion solution, the amount of copper nitrate is 10% by...
Embodiment 2
[0051] Embodiment 2—preparation of catalyst 2
[0052] Prepare 63mL of a mixed aqueous solution containing copper nitrate, iridium trichloride, ammonium phosphate, and zirconium nitrate as the impregnating liquid. In the impregnating liquid, the amount of copper nitrate is 8% by weight calculated as copper element, and the amount of iridium trichloride is calculated as iridium element The amount of ammonium phosphate was 0.5% by weight, the amount of ammonium phosphate was 3% by weight as phosphorus element, and the amount of zirconium nitrate was 0.5% by weight as zirconium element. At room temperature, take 100g of titanium oxide-alumina composite oxide (A-1), impregnate it with the previously prepared impregnation solution, dry it at 120°C for 4 hours, then calcinate it at 500°C for 4 hours, take it out and cool it to room temperature . The catalyst prepared in this way is designated Catalyst 2.
Embodiment 3
[0053] Embodiment 3—preparation of catalyst 3
[0054] Prepare 63mL of a mixed aqueous solution containing copper nitrate, iridium trichloride, ammonium phosphate and nickel nitrate as the immersion solution, in the immersion solution, the amount of copper nitrate is 7% by weight calculated as copper element, and the amount of iridium trichloride is calculated as iridium element The amount of ammonium phosphate was 0.3% by weight, the amount of ammonium phosphate was 2% by weight as phosphorus element, and the amount of nickel nitrate was 3% by weight as nickel element. At room temperature, take 100g of titanium oxide-alumina composite oxide (A-1), impregnate it with the previously prepared impregnation solution, dry it at 120°C for 4 hours, then calcinate it at 500°C for 4 hours, take it out and cool it to room temperature . The catalyst prepared in this way is designated Catalyst 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com