Fluorescent probe used for detecting glutathione as well as preparation method and application thereof
A technology of glutathione and fluorescent probes, which is applied in the fields of fluorescence/phosphorescence, chemical instruments and methods, biological testing, etc., can solve problems such as the difficulty in realizing specific detection of glutathione, and achieve easy promotion and application, excellent Responsive sensitivity, low-cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
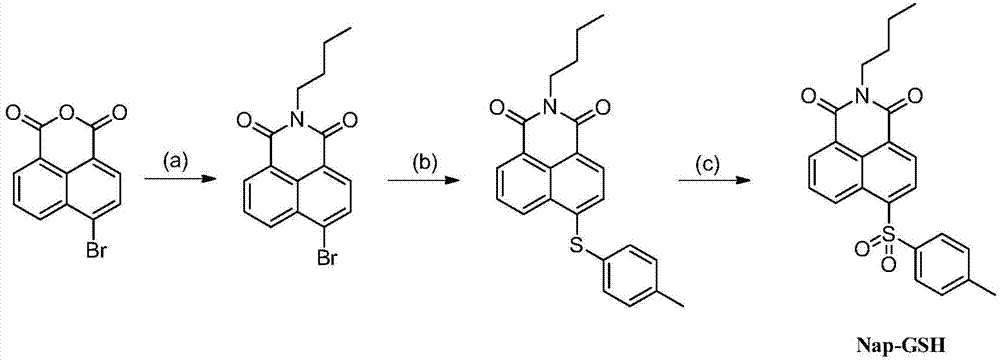
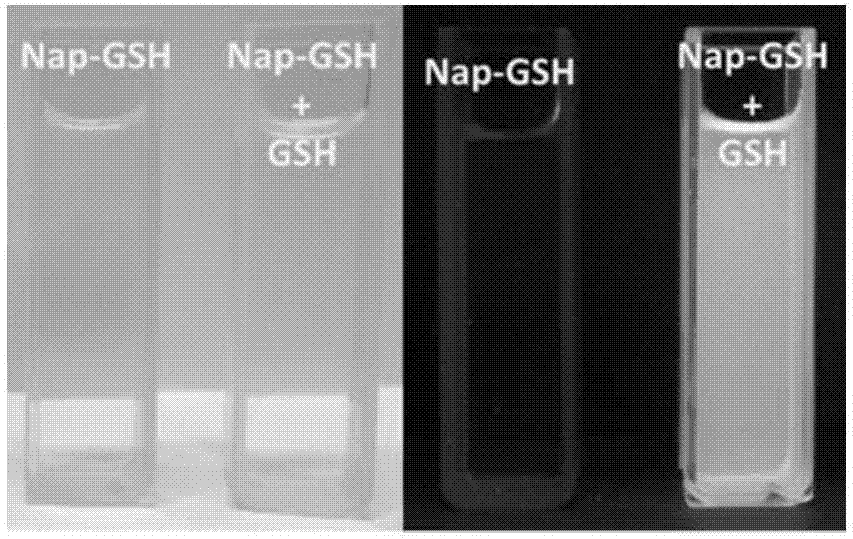
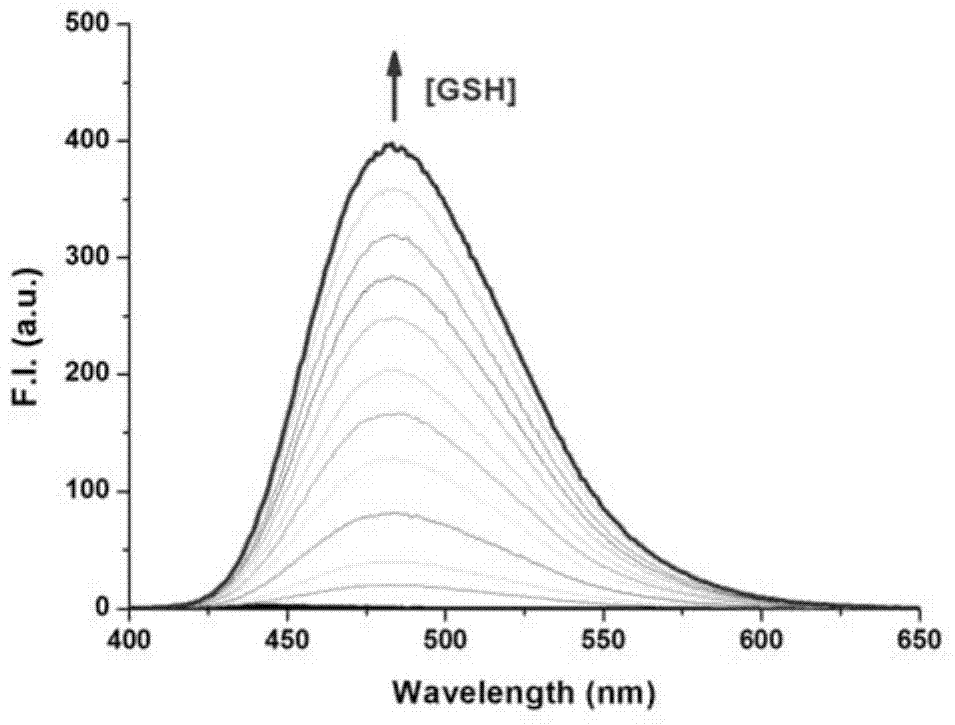
[0067] Embodiment 2, the preparation of fluorescent probe Nap-GSH
[0068] Step a): Under an inert atmosphere, add 5.00g of 4-bromo-1,8-naphthalene dicarboxylic anhydride to 100mL of anhydrous methanol, then inject 5.0mL of n-butylamine, and reflux at a reaction temperature of 120°C 10 hours. After the reaction was complete, needle-like crystals precipitated after standing overnight, filtered and washed three times with cold ethanol to obtain 4.40 g of intermediate N-n-butyl-4-bromo-1,8-naphthalimide (yield 84%).
[0069] Step b): Under an inert atmosphere, add 1.00g of N-butyl-4-bromo-1,8-naphthalimide and 3.94g of p-methylthiophenol into a 50mL three-necked flask, inject 20mL of butanol and 2.5 mL of diisopropylethylamine was refluxed for 10 hours at a reaction temperature of 140°C. After the reaction is complete, the reaction solution is poured into 200mL of ice water, a large amount of solids precipitate out, filtered, washed, and dried in vacuo to obtain N-n-butyl-4-(p-...
Embodiment 3
[0073] Embodiment 3, the preparation of fluorescent probe Nap-GSH
[0074] Step a): Under an inert atmosphere, add 5.00g of 4-bromo-1,8-naphthalic anhydride to 100mL of anhydrous n-propanol, then inject 7.5mL of n-butylamine, at a reaction temperature of 80°C, Reflux for 5 hours. After the reaction was complete, needle crystals precipitated after standing overnight, filtered and washed three times with cold ethanol to obtain 4.60 g of intermediate N-n-butyl-4-bromo-1,8-naphthalimide (yield 87%).
[0075] Step b): Under an inert atmosphere, add 1.00g of N-n-butyl-4-bromo-1,8-naphthalimide and 0.38g of p-methylthiophenol into a 50mL three-necked flask, inject 20mL of diethyl Glycol monomethyl ether and 2.9 g of potassium carbonate were refluxed for 24 hours at a reaction temperature of 140°C. After the reaction is complete, the reaction solution is poured into 200mL of ice water, a large amount of solids precipitate out, filtered, washed, and dried in vacuo to obtain N-n-butyl...
Embodiment 4
[0079] Embodiment 4, the preparation of fluorescent probe Nap-GSH
[0080] Step a): Under an inert atmosphere, add 5.00g of 4-bromo-1,8-naphthalene dicarboxylic anhydride to 100mL of anhydrous n-butanol, then inject 10.0mL of n-butylamine, and at a reaction temperature of 70°C, The reaction was refluxed for 1 hour. After the reaction was complete, needle-like crystals precipitated after standing overnight, filtered and washed three times with cold ethanol to obtain 4.84 g of intermediate N-n-butyl-4-bromo-1,8-naphthoimide (yield 88%).
[0081] Step b): Under an inert atmosphere, add 1.00g of N-n-butyl-4-bromo-1,8-naphthalimide and 2.87g of p-methylthiophenol into a 50mL three-necked flask, inject 20mL of ethanol and 3.5 g of potassium hydroxide was refluxed for 1 hour at a reaction temperature of 100°C. After the reaction is complete, the reaction solution is poured into 200mL of ice water, a large amount of solids precipitate out, filtered, washed, and dried in vacuo to obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com