Recombinant plasmid, recombinant baculovirus prepared from the same and application of virus in vaccine preparation
A technology of recombinant baculovirus and recombinant plasmid, applied in application, virus/phage, resistance to vector-borne diseases, etc., can solve the problems of high cost, low expression, rejection and other problems, achieve high yield, high application value, cost reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
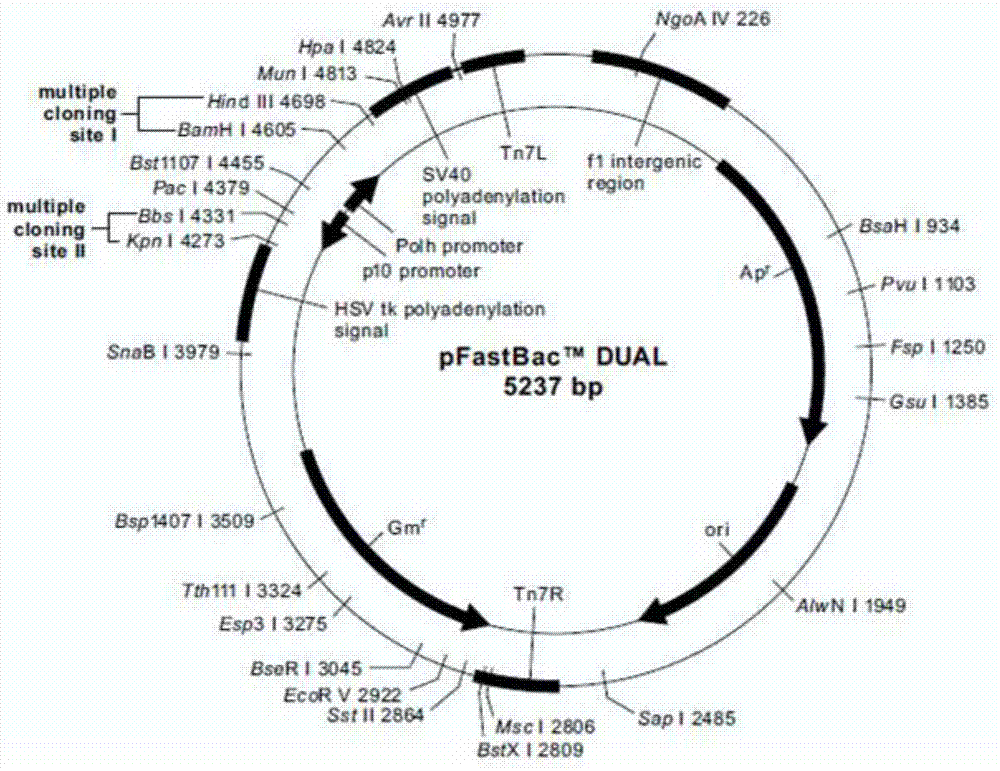
[0049] Embodiment 1: Construction of recombinant plasmid pFastBacDual-CMV-Pph-SP-TM
[0050] According to the known sequences of CMV, Pph, SP, and TM, add Xba I restriction site and Xho I restriction site between SP and TM nucleotide sequence, add KpnI restriction site before CMV-F, TM-R Afterwards, a HindIII restriction site was added to synthesize the CMV-Pph-SP-TM sequence (as shown in SEQ ID NO: 1), and primers CMV-F (as shown in SEQ ID NO: 2), TM-R (as shown in SEQ ID NO: 2) and TM-R (as shown in shown in SEQ ID NO: 3). Primers are as follows:
[0051] CMV-F5'-CGG GGTACC TAGTTATTAATAGT-3'
[0052] TM-R5'-CCC AAGCTT TTAATATTGTCTAC-3'
[0053] Wherein the underline is the restriction site.
[0054] The target fragment was amplified by PCR using the synthesized CMV-Pph-SP-TM sequence as a template and CMV-F and TM-R as upstream and downstream primers. The reaction system of PCR is:
[0055]
[0056]
[0057] After each component was mixed, put it into a PCR m...
Embodiment 2
[0058] Example 2: Construction of the recombinant transposable plasmid pFstBacDual-CMV-Pph-SP-Pvs25-TM
[0059] The target gene Pvs25 was amplified by PCR using the T-Pvs25-Pvs48 plasmid as a template, and Pvs25-F (as shown in SEQ ID NO: 4) and Pvs25-R (as shown in SEQ ID NO: 5) as upstream and downstream primers. Primers are as follows:
[0060] Pvs25-F5'-CGC TCTAGA ATGGAAGAAAAAAAT-3'
[0061] Pvs25-R5'-CCG CTCGAG AAGGCATACATTT-3'
[0062] Wherein the underline is the restriction site.
[0063] The reaction system of PCR is:
[0064]
[0065]
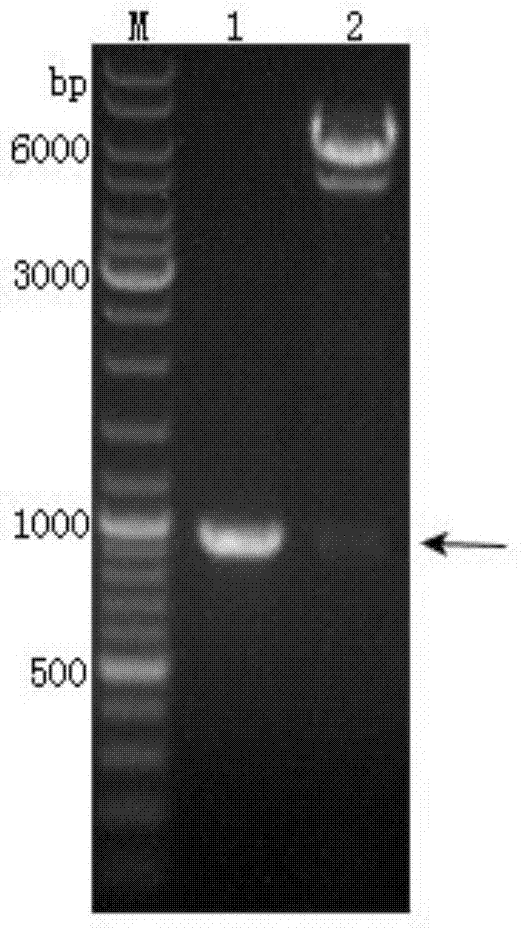
[0066] After each component was mixed, put it into a PCR machine, PCR reaction parameters: 95°C pre-denaturation for 5 min, 95°C denaturation for 1 min, 56°C annealing for 30 s, 72°C extension for 30 s, 35 cycles, 72°C stop extension for 10 min. After the reaction was completed, the amplified product fragment was identified by electrophoresis, and the target fragment was 414bp in size, and the target fragment was recovere...
Embodiment 3
[0067] Embodiment 3: the acquisition of Bombyx mori recombinant baculovirus BmPvs25
[0068] The recombined transposable plasmid pFastBacDual-CMV-Pph-SP-Pvs25-TM, which was successfully identified for recombination, was transformed into Escherichia coli DH10Bac competent cells containing the baculovirus shuttle vector Bacmid, in the presence of kanamycin, gentamicin, tetracycline, X-gal and IPTG were cultured on the LB culture plate (operated according to the instructions), and the blue and white spots were screened after homologous recombination by transposition. After 48 hours of dark culture, the white spots were picked, and the white spots continued to be treated with tetracycline and kanamycin. , Gentamicin, X-gal and IPTG in the LB culture solution of shake culture 48h after using isopropanol to extract recombinant baculovirus genomic DNA, use M13 universal primer (M13-F as shown in SEQ ID NO: 6 , M13-R as shown in SEQ ID NO: 7), Pvs25-F and Pvs25-R identified the insert...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com