Method for synthesizing spherical manganese carbonate by use of ionic liquid
A technology of ionic liquid and manganese carbonate, which is applied in the production of chemical instruments and methods, manganese compounds, and bulk chemicals, can solve the problems of less research on product morphology control, and achieve low operating costs, low energy consumption, and synthetic processes simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
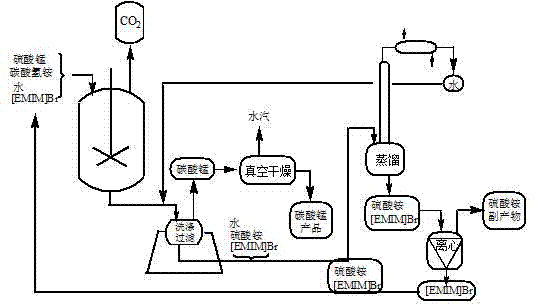
[0024] Example 1 Step 1: Add ionic liquid (1-ethyl-3-methylimidazolium bromide [EMIM] Br), manganese sulfate monohydrate, ammonium bicarbonate, and water into a beaker at a mass ratio of 6:2:1:10, at 20°C After stirring and reacting for 20 minutes, filter, wash and separate to obtain manganese carbonate, and collect carbon dioxide simultaneously;
[0025] In the second step, the product manganese carbonate is dehydrated and vacuum-dried at 60°C for 6 h (vacuum degree is 0.0868MPa) to obtain a spherical manganese carbonate product.
[0026] In the third step, the washed filtrate is subjected to simple distillation to remove water, and the solid ammonium sulfate, manganese sulfate mixture and ionic liquid are centrifuged again. The ionic liquid, ammonium sulfate and manganese sulfate mixture were recovered separately.
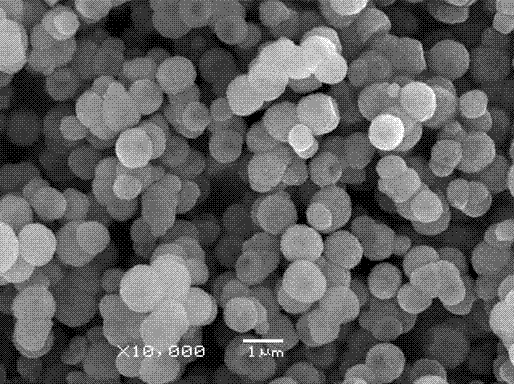
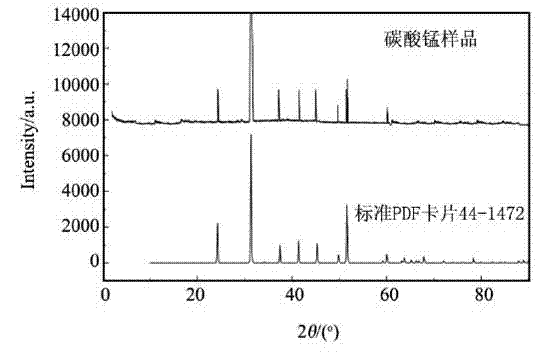
[0027] The product synthesized under this condition is characterized by morphology, structure and particle size, and the product is D 50 =1.256μm, spherical ...
Embodiment 2
[0028] Example 2 In the first step, ionic liquid ([EMIM]Br), manganese sulfate monohydrate, ammonium bicarbonate, and water are added into a beaker at a mass ratio of 7:2:1:9, stirred and reacted at 20°C for 20 minutes, filtered and washed with water to obtain manganese carbonate, while collecting carbon dioxide;
[0029] In the second step, the product manganese carbonate is dehydrated and vacuum-dried at 60°C for 6 h (vacuum degree is 0.0868MPa) to obtain the manganese carbonate product.
[0030] In the third step, the washed filtrate is subjected to simple distillation to remove water, and the solid ammonium sulfate, manganese sulfate mixture and ionic liquid are centrifuged again. The ionic liquid, ammonium sulfate and manganese sulfate solid mixtures were recovered separately.
[0031] The product synthesized under this condition is characterized by morphology, structure and particle size, and the product is D 50 =1.125μm, spherical particles of manganese carbonate wi...
Embodiment 3
[0032] Example 3 In the first step, ionic liquid ([EMIM]Br), manganese sulfate monohydrate, ammonium bicarbonate, and water are added into a beaker at a mass ratio of 8:2:1:8, stirred and reacted at 20°C for 20 minutes, filtered, washed and separated to obtain manganese carbonate, while collecting carbon dioxide;
[0033] In the second step, the product manganese carbonate is dehydrated and vacuum-dried at 60°C for 6 h (vacuum degree is 0.0868MPa) to obtain the manganese carbonate product.
[0034] In the third step, the washed filtrate is subjected to simple distillation to remove water, and the solid ammonium sulfate, manganese sulfate mixture and ionic liquid are centrifuged again. The ionic liquid, ammonium sulfate and manganese sulfate solid mixtures were recovered separately.
[0035] The product synthesized under this condition is characterized by morphology, structure and particle size, and the product is D 50 =1.030μm, spherical particles of manganese carbonate wi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| decomposition temperature | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com