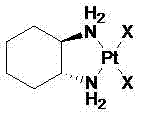
Industrial production method of miriplatin
A production method, the technology of rice platinum, applied in the direction of chemical instruments and methods, organic chemistry, platinum group organic compounds, etc., can solve the problems of harsh process conditions and low solubility, and achieve the advantages of simple product separation, low cost and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
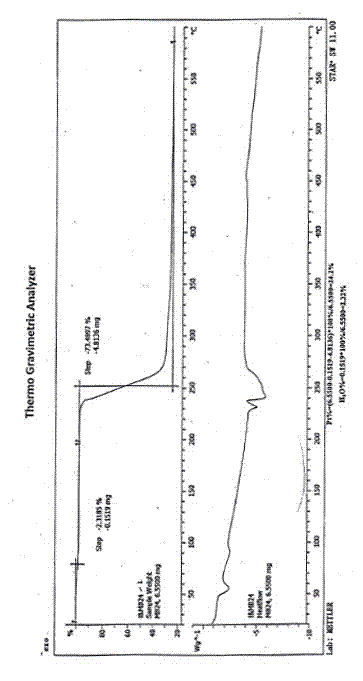
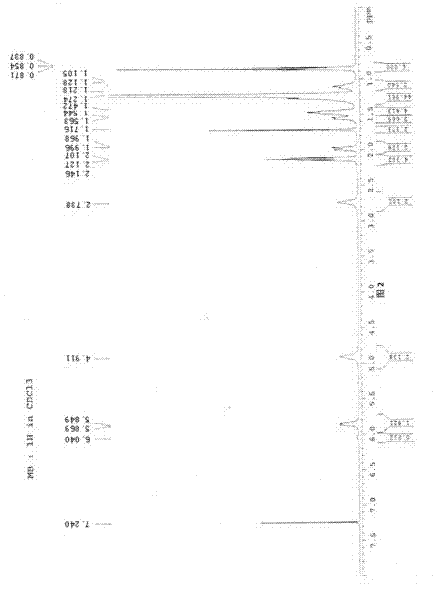
Image
Examples
Embodiment 2
[0045] Preparation of cis-[(1R,2R)-1,2-cyclohexanediamine-diiodo]platinum (Ⅱ):
[0046] Add 300mL of distilled water to 10g of potassium chloroplatinite, dissolve and filter, and add the filtrate to a 1000mL four-necked bottle. Dissolve 32g of potassium iodide in 150mL of distilled water, and then add it to the above reaction flask. After the mixture is stirred for 30 minutes, add 2.7g of (1R,2R)-1,2-cyclohexanediamine distilled aqueous solution (200mL of distilled water) dropwise. , reacted in the dark at 20-25°C for 20 hours, a dark yellow solid precipitated, filtered, washed with 50mL of ice water, 50mL of ethanol, and 50mL of diethyl ether in sequence, and dried to obtain 11.1g of a yellow solid, with a yield of 82%.
preparation Embodiment 1
[0048] Preparation of Miplatin:
[0049] 2.70g cis-[(1R,2R)-1,2-cyclohexanediamine-dichloro]platinum (Ⅱ), 2.41g silver nitrate and 50mL water, stirred and reacted at room temperature for 24 hours in the dark, filtered and filtered Silver chloride to obtain cis-[(1R,2R)-1,2-cyclohexanediamine-dihydrate] platinum aqueous solution for future use.
[0050] Add 4.87g of myristic acid, 2.98g of ammonia water (25% by weight) and 10mL of distilled water into a 250mL reaction bottle, heat to 40°C, after clarification, add the above-prepared cis-[(1R,2R)-1, 2-Cyclohexanediamine-dihydrate] platinum (II) filtrate. After dropping, the reaction system was reacted at 40° C. in the dark for 36 hours. Add 30mL of ethanol to the system at 40°C, stir and disperse for 3 hours, and filter to obtain 4.7g of platinum, with a yield of 84.7%, HPLC content of 99.3%, and chiral HPLC of 100%.
[0051] Example 2:
[0052] Preparation of Miplatin:
[0053] 4.0g cis-[(1R,2R)-1,2-cyclohexane...
Embodiment 3
[0056] Preparation of Miplatin:
[0057] 2.70g cis-[(1R,2R)-1,2-cyclohexanediamine-dichloro]platinum (Ⅱ), 2.41g silver nitrate and 50mL water, stirred and reacted at room temperature for 24 hours in the dark, filtered and filtered Silver chloride to obtain cis-[(1R,2R)-1,2-cyclohexanediamine-dihydrate] platinum (II) aqueous solution for future use.
[0058] Add 3.24g of myristic acid, 0.80g of potassium hydroxide and 10mL of distilled water into a 250mL reaction bottle, heat to 50°C, after clarification, add the above-prepared cis-[(1R,2R)-1,2-cyclohexanedi Amine-dihydrate] platinum (II) filtrate. After dropping, the reaction system was reacted at 50° C. in the dark for 24 hours. Add 110mL of ethanol to the system at 50°C, stir and disperse for 1 hour, and filter to obtain 4.3g of platinum, with a yield of 77.5%, HPLC content of 99.3%, and chiral HPLC of 100%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com