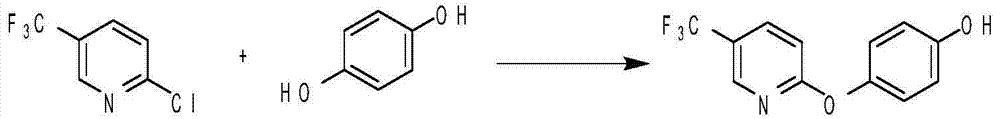Method for preparing 4-substituted oxyphenol compound
An oxyphenol and compound technology, applied in the field of organic synthesis, can solve the problems of poor selectivity for synthesizing 4-substituted oxyphenol, difficult recovery of solvent and hydroquinone, complicated process, etc., and avoid side reactions and reaction steps. Short, highly selective effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 Synthesis of 4-(5-trifluoromethylpyridyl-2-oxygen)phenol
[0035] The synthetic reaction formula is:
[0036]
[0037] The specific synthesis is as follows: add 6.0g of sodium hydroxide, 11.1g of hydroquinone, 8.0g of sodium bisulfite and 50g of DMF to the reaction bottle, then raise the temperature, and add dropwise 9.2g of 2-chloro-5-trifluoroform under temperature control at 90°C Basepyridine, add dropwise in about 1 hour, after dropping, keep warm at 90°C for 5 hours, after the conversion of raw materials is complete, distill and concentrate the reaction solution under reduced pressure, add 100ml of toluene after completion, stir for 1 hour, filter, and recover hydroquinone. Add 100ml of 5% sodium hydroxide aqueous solution to the toluene phase to extract twice, separate the water phase, add hydrochloric acid dropwise at a temperature of 25-30°C to acidify to pH 5-6, stir for 30 minutes, filter and dry to obtain 12.5g of 4-(5-tri Fluoromethylpyridyl-2-...
Embodiment 2
[0038] Example 2 Synthesis of 4-(5-trifluoromethylpyridyl-2-oxygen)phenol
[0039] After adding 86.8g of potassium hydroxide, 166.5g of hydroquinone, 88.5g of sodium bisulfite and 500g of DMF into the reaction flask, the temperature was raised, and 92g of 2-chloro-5-trifluoromethylpyridine was added dropwise at a temperature of 50°C, about 1 The dropwise addition was completed within 1 hour. After the dropwise completion, the reaction was carried out at 90°C for 5 hours. After the conversion of the raw materials was completed, the reaction solution was concentrated by distillation under reduced pressure. After the completion, 1000ml of toluene was added, stirred for 1 hour, filtered, and hydroquinone was recovered. Add 1000ml of 5% sodium hydroxide aqueous solution to the toluene phase to extract twice, separate the water phase, add hydrochloric acid dropwise at a temperature of 25-30°C to acidify to pH 5-6, stir for 30 minutes, filter and dry to obtain 128.8g of 4-(5-tri Fluo...
Embodiment 3
[0040] Example 3 Synthesis of 4-(5-trifluoromethylpyridyl-2-oxygen)phenol
[0041] The operation method is the same as in Example 1, and the solvent is replaced with DMSO to obtain a product content of 97.8% and a yield of 96.9%. Recover hydroquinone 5.3g, content 94.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com