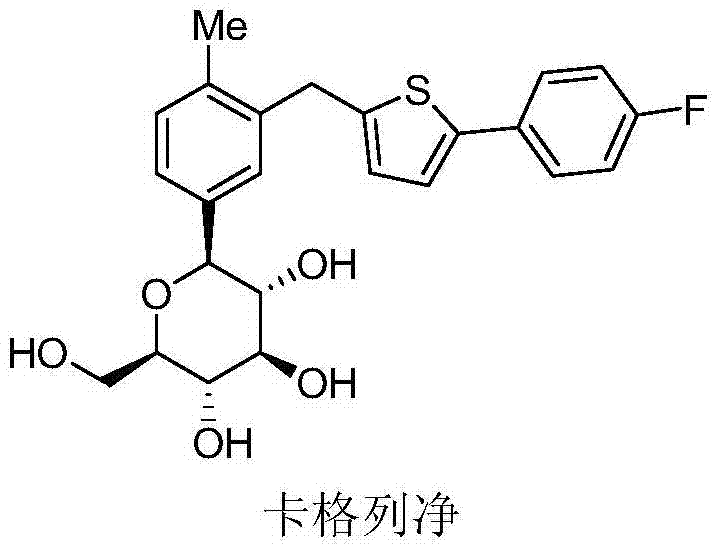
Preparation method of canagliflozin hemihydrate and monocrystal thereof
A technology of canagliflozin hemihydrate and single crystal, which is applied in organic chemistry and other fields, and can solve problems such as difficult quality control, inconvenient operation, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
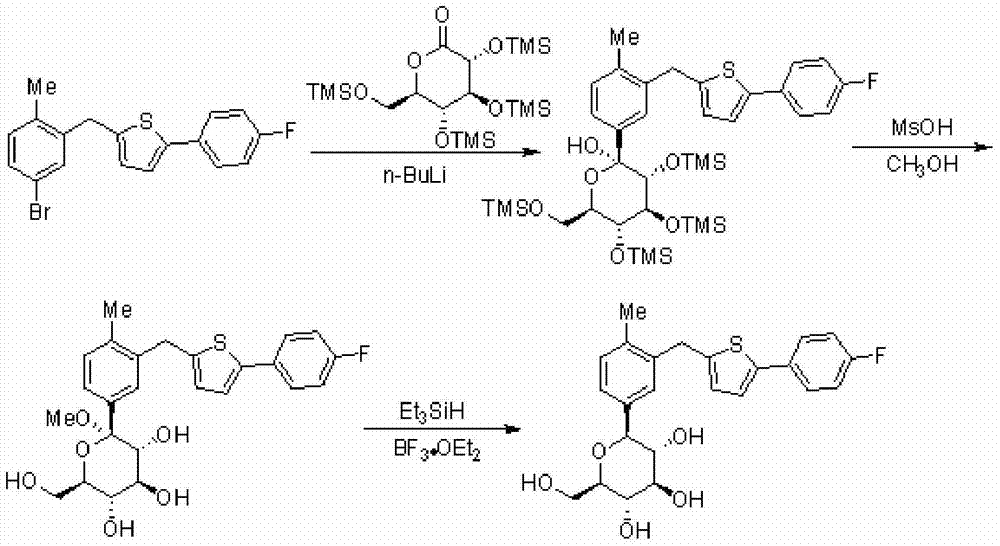
Embodiment 1
[0058] Synthesis of [5-(4-fluorophenyl)thiophen-2-yl](5-iodo-2-methylphenyl)methanone (II)
[0059]
[0060] The starting material 5-iodo-2-methylbenzoic acid (100.06g, 0.38mol) was added to 400ml of dichloromethane, stirred at room temperature, and N,N-dimethylformamide (1.89g, 0.026mol) was added dropwise , and then dropwise added oxalyl chloride (74.03 g, 0.58 mol), and stirred at room temperature for about 2 hours to complete the reaction. The reaction solution was evaporated under reduced pressure to remove the solvent to obtain a white solid for use.
[0061] Add aluminum trichloride (60.81g, 0.45mol) into 400ml of dichloromethane, stir at room temperature, then add 2-(4-fluorophenyl)thiophene (57.03g, 0.32mol), and then add the white solid obtained above, React at 20-25°C for 2 hours. After the reaction is complete, cool the reaction liquid to -10°C, then slowly add 200ml of water to quench, add 400ml of dichloromethane to extract twice, wash once with saturated sali...
Embodiment 2
[0075] Preparation of single crystal of canagliflozin hemihydrate (V)
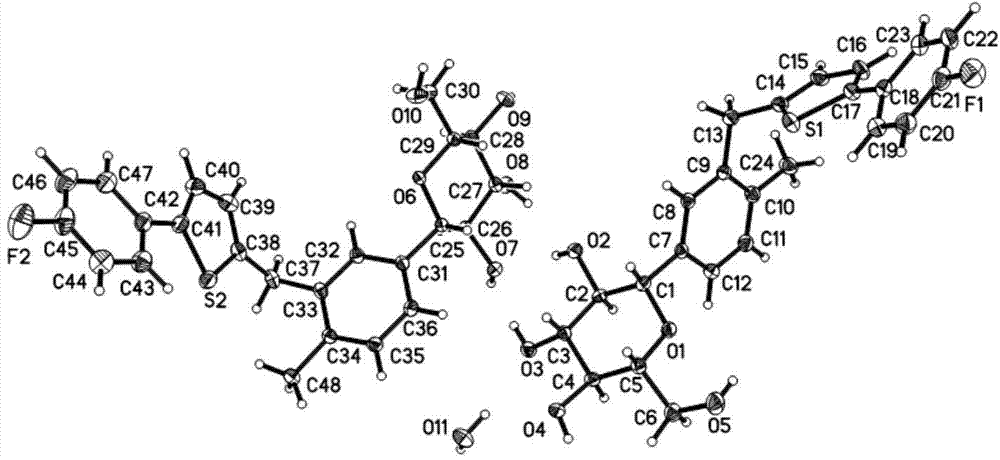
[0076] Dissolve canagliflozin hemihydrate (1.52g) in methanol (10.00ml) at a temperature of 18°C to 20°C, then add water (11.00ml) dropwise, mix well, use solvent evaporation method, crystal Grow to 18-20 days, can obtain the single crystal of canagliflozin hemihydrate of good quality, obtain single crystal data through X-ray diffraction as shown in Table 1, its single crystal structure model is as follows figure 1 shown.
[0077] Table 1: Single crystal data of canagliflozin hemihydrate
[0078]
[0079]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com