Alizarin aminophosphonate derivatives and their synthesis method and use
A technology of alizarin aminophosphonate and aminophosphonate, which is applied in the field of alizarin aminophosphonate derivatives and their synthesis, and can solve problems such as no public reports of α-aminophosphonate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
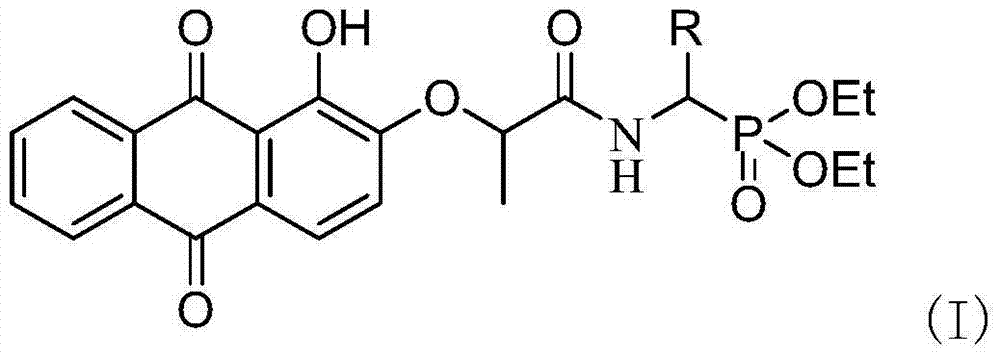
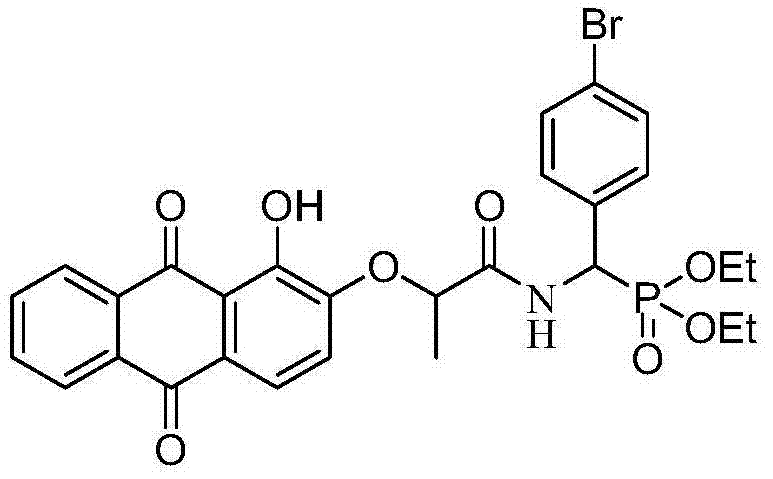
[0059] Example 1: O, O'diethyl{[2-(1-hydroxy-9,10-dioxo-9,10-dihydroanthracene-2-oxymethyl)propionylamino](4-bromo Synthesis of diethyl phenyl)methyl}phosphonate (a)
[0060] 1) Synthesis of diethyl α-O, O'diethylamino(4-bromophenyl)methylphosphonate
[0061] Add p-bromobenzaldehyde, ammonium acetate and diethyl phosphite (2:1:1 molar ratio) in a round bottom flask, stir and react at 80°C for 10 hours, then add the amount of diethyl ether into the round bottom flask is 1.2 times the amount of p-bromobenzaldehyde), concentrated hydrochloric acid was added under ice bath to make the reaction system acidic (pH=6) and stirred for 3h, the reaction system was extracted with water, the aqueous layer was collected and extracted with ether to remove organic impurities, Collect the water layer again, and in the water layer of collection, add the sodium hydroxide solution that mass concentration is 10% and adjust its pH to be 9, extract with ethyl acetate, collect the organic layer, the...
Embodiment 2
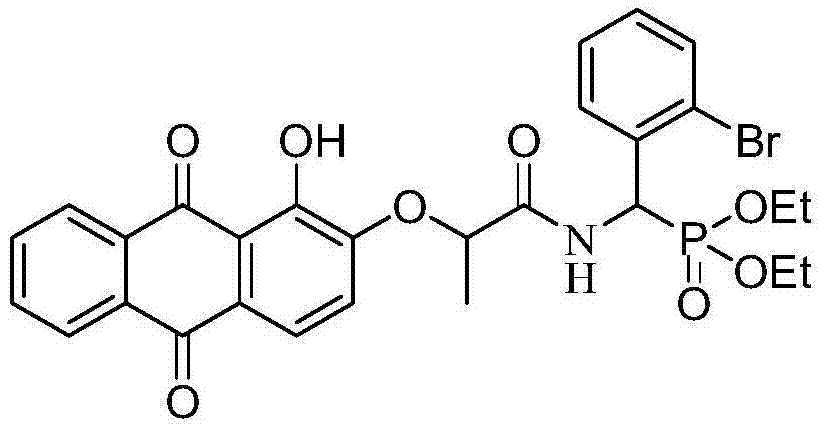
[0069] Example 2: O, O'diethyl{[2-(1-hydroxy-9,10-dioxo-9,10-dihydroanthracene-2-oxymethyl)propionylamino](2-bromo Synthesis of diethyl phenyl)methyl}phosphonate (b)
[0070] 1) Synthesis of diethyl α-O, O'diethylamino(2-bromophenyl)methylphosphonate
[0071] Synthesize according to the method and conditions described in step 1) in Example 1, except that p-bromobenzaldehyde is replaced with o-bromobenzaldehyde.
[0072] 2) Synthesis of target product:
[0073] 2.1) with embodiment 1;
[0074] 2.2) In a round bottom flask, dissolve 1 mmol 2-(1-hydroxy-9,10-dioxo-9,10-dihydroanthracen-2-yloxy)propionic acid in 10 mL N,N-dimethyl In formamide, add 0.1 mmol catalyst 1-hydroxybenzotriazole dropwise under ice bath, stir at room temperature for 3 min, then add 1.5 mmol condensing agent 1-(3-dimethylaminopropyl)-3-ethylcarbodiethylene Amine hydrochloride, continue to stir for 13min; Add dropwise 1mmolα-O, O' diethylamino (2-bromophenyl) methylphosphonic acid diethyl ester (with 5m...
Embodiment 3
[0079] Example 3: O, O'diethyl{[2-(1-hydroxy-9,10-dioxo-9,10-dihydroanthracene-2-oxymethyl)propionylamino](3-bromo Synthesis of diethyl phenyl)methyl}phosphonate (c)
[0080] 1) Synthesis of diethyl α-O, O'diethylamino(3-bromophenyl)methylphosphonate
[0081] Synthesize according to the method and conditions described in step 1) in Example 1, except that m-bromobenzaldehyde is used instead of p-bromobenzaldehyde.
[0082] 2) Synthesis of target product:
[0083] 2.1) with embodiment 1;
[0084] 2.2) In a round bottom flask, dissolve 1mmol of 2-(1-hydroxy-9,10-dioxo-9,10-dihydroanthracen-2-yloxy)propionic acid in 10mL of methanol, under ice bath Add 0.05 mmol of catalyst 1-hydroxybenzotriazole dropwise, stir at room temperature for 5 min, then add 2 mmol of condensing agent 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, and continue stirring for 3 min; Then add 1mmol α-O, O'diethylamino (3-bromophenyl) methylphosphonic acid diethyl ester (dissolved in 20mL of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com