Glucose isomerase and gene, mutant, engineering bacteria and application thereof
A technology of glucose isomerase and genetically engineered bacteria, applied in the field of genetic engineering, can solve the problem of low reaction temperature, achieve high yield, simple process, and easy industrial application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Gene synthesis of glucose isomerase
[0044] The gene of glucose isomerase is derived from the ctg00047 sequence fragment of the whole genome shotgun sequencing of Thermoanaerobacter ethanolicus CCSD1, wherein the bases from 7706bp to 9022bp are the sequence encoding the glucose isomerase gene (GenBank No.EEU61835.1, GI 256748793). In order to express the protein with His-tag after the gene is connected to the vector pET-28b, its stop codon was excised, and its sequence and common restriction endonuclease were compared with the codon preference of B. subtilis 168 as a reference The sequences of the recognition sites BamH I, Xho I, Pst I, Hind III and Nco I were optimized. The sequence of the newly designed glucose isomerase gene (tegi) is shown in SEQ ID NO.1. The gene synthesis work was entrusted to Shanghai Xuguan Completed by Biotechnology Development Co., Ltd., the gene was synthesized and connected to the cloning vector pES.
Embodiment 2
[0045] Example 2: Construction of glucose isomerase recombinant Bacillus subtilis
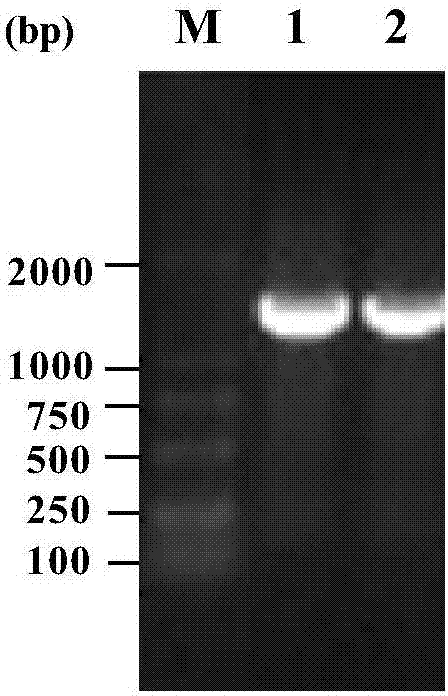
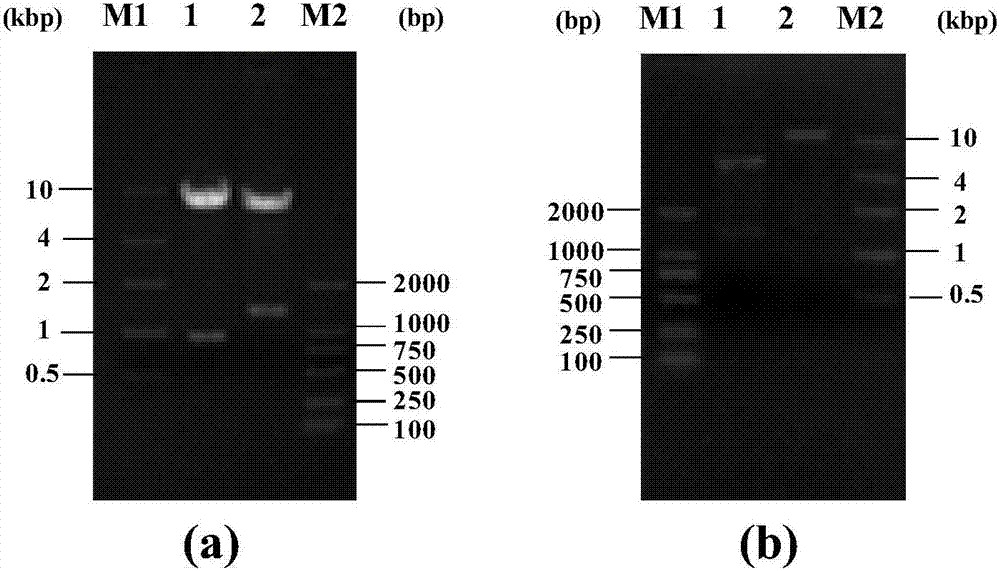
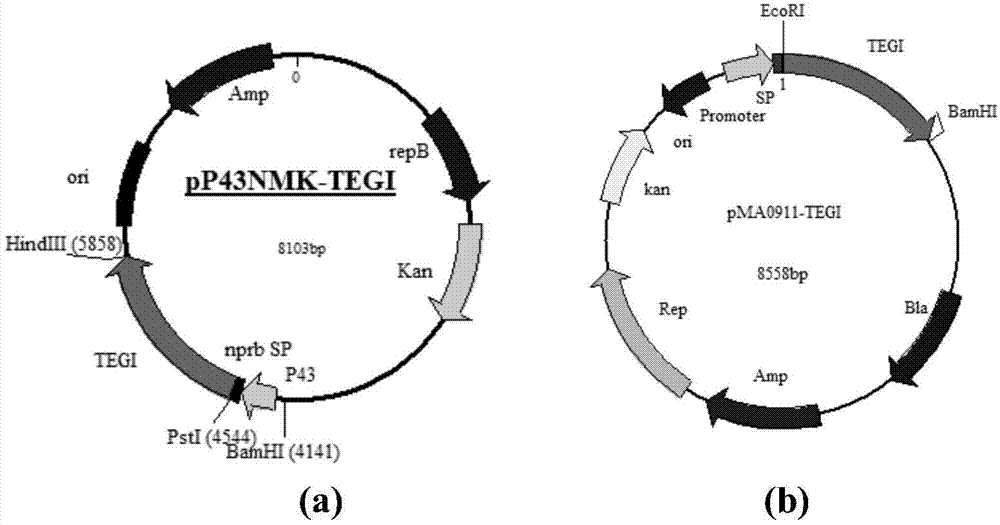
[0046] On the basis of Example 1, primers were designed respectively for different Bacillus subtilis expression vectors, using PCR technology, with the synthetic nucleotide sequence (shown in SEQ ID NO.1) as template, glucose isomerase gene ( tegi) for amplification. For expression vector pP43NMK, design primer tegi-NMK-F: 5'-AAAA CTGCAG ATGGAATACTTCAAAAACG-3' and tegi-NMK-R:5'-CCC AAGCTT TTATTCAGAGAAAAGGTATTGG-3', and introduce Pst I and Hind III restriction enzyme sites respectively; for expression vector pMA0911, design primer tegi-MA-F: 5'-CCG GAATTC ATGGAATACTTCAAAAACG-3' and tegi-MA-R:5'-CGC GGATCC TTATTCAGAGAAAAGGTATTGG-3', and introduced EcoR I and BamH I restriction enzyme sites respectively.
[0047]The PCR reaction system (50 μL) was: 5 μL of 10×Pfu PCR buffer, 8 μL of dNTP Mixture; 1 μL of template DNA; 2 μL of upstream and downstream primers; 0.5 μL of Pfu DNA polymerase; 3...
Embodiment 3
[0050] Embodiment 3: the enzyme activity assay of recombinant Bacillus subtilis
[0051] Recombinant bacteria B. subtilis WB800 / pP43NMK-tegi and B. subtilis WB800 / pMA0911-tegi after enzyme digestion and sequencing verification were inoculated into 50 mL LB liquid medium (containing kanamycin 40 μg / mL), 37 °C , 150r / min shaking culture to OD 600 =0.8~1.0; the culture solution was inoculated into fresh 100mL LB liquid medium containing 40μg / mL kanamycin with 2% (v / v) inoculation amount, and cultured with shaking at 37°C and 150r / min for 10~24h.
[0052] Take the cell culture solution, centrifuge at 1000r / min for 5min, take the cell pellet and supernatant respectively, add 20μL SDS buffer solution to mix, heat in a boiling water bath for 5min, and perform SDS-PAGE analysis, and use the empty host without carrier as a control, As a result, no obvious glucose isomerase protein band was found; correspondingly, no enzyme activity of glucose isomerase was detected. It indicated that...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com