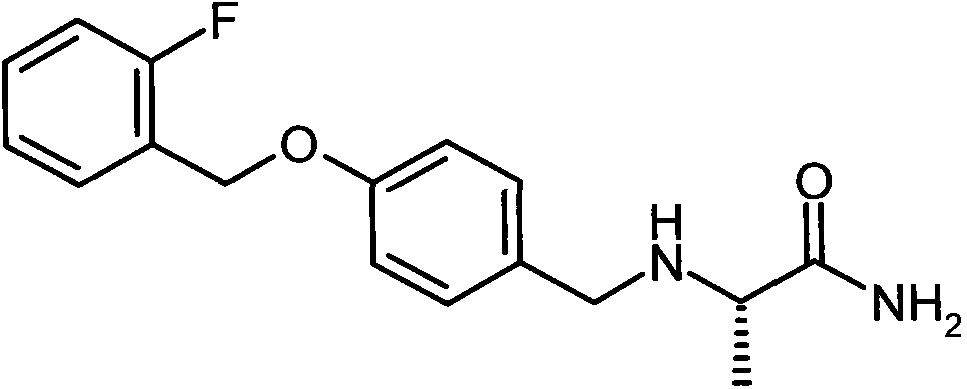
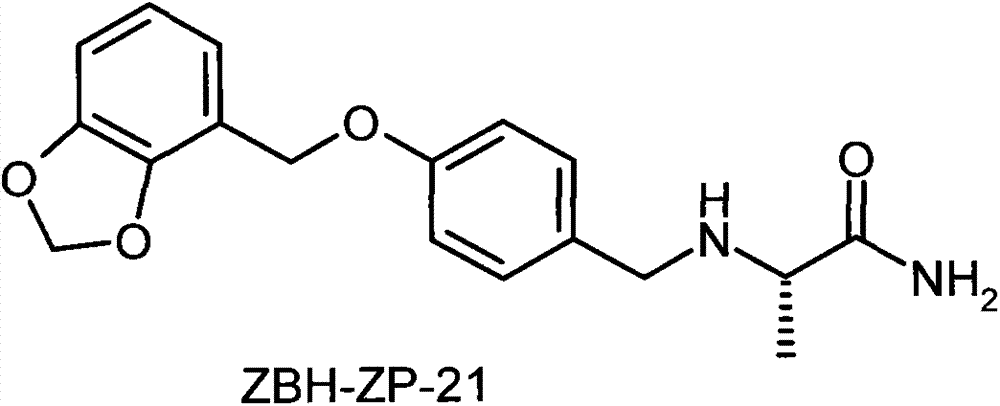
Analgesic active compounds and their medicinal uses
A compound and toxicity technology, applied in the direction of active ingredients of heterocyclic compounds, antipyretics, organic chemistry, etc., can solve problems such as side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Synthesis of Example 1 (S)-2-(4-(benzo[1,3]dioxacyclo-5-oxymethyl)benzyl)amino-propionamide (1)
[0049]
[0050] 1.1 Synthesis of 4-hydroxymethylbenzaldehyde (17)
[0051] Take 5.0g p-phthalaldehyde (37.5mM) in a round bottom bottle, add 60ml ethanol and 90ml tetrahydrofuran to dissolve, add NaBH under ice-cooling 4 0.43g (9.3mM), reacted for 6h, and monitored the reaction by TLC. Stop the reaction, add 2M hydrochloric acid solution to quench, reduce the pH to about 5, rotary evaporate the solution, add water and ethyl acetate to dissolve the residue, separate the liquid, extract the aqueous phase with ethyl acetate 2 to 3 times, combine the organic phase, and saturate Wash with brine and dry over anhydrous sodium sulfate. The desiccant was filtered off, the solvent was distilled off under reduced pressure, and the residue was separated by silica gel column chromatography, the eluent was petroleum ether: ethyl acetate = 3:1, and the required components were collec...
Embodiment 2
[0056] Synthesis of Example 2 (S)-2-(4-(benzo[1,3]dioxacyclo-5-oxomethyl)benzyl)amino-acetamide (2)
[0057]
[0058] Dissolve 1.2g (11.0mM) glycinamide hydrochloride, 0.5g (8.0mM) sodium cyanoborohydride, 1.0g3A molecular sieve and 1ml triethylamine in 40ml methanol, stir at room temperature for 15min, and quickly add 2.56g compound 19 (10.0mM), heated to 40°C and stirred for 3h. After stopping the reaction, filter, distill off the solvent, add water and ethyl acetate to dissolve, separate the layers, wash with saturated brine and dry for 8 hours. The desiccant was filtered off, the solvent was distilled off under reduced pressure, and the residue was separated by silica gel column chromatography. The eluent was dichloromethane: anhydrous methanol: ammonia water = 80:1:0.1, and the required components were collected to obtain compound 2 0.8 g of white solid, yield 25.7%. MS (ESI) m / z: 315.6 (M+H + ); Proton NMR spectrum: 1H-NMR (400MHz, DMSO-d4): 1H-NMR (400MHz, DMSO-d4...
Embodiment 3
[0059] Synthesis of Example 3 (S)-2-(4-(benzo[1,3]dioxacyclo-4-oxymethyl)benzyl)amino-acetamide (3)
[0060]
[0061] 3.1 Synthesis of 4-(benzo[1,3]-dioxacyclo-4-oxomethyl)benzaldehyde (20)
[0062] Dissolve 3.1g o-hydroxypiperone (22.7mM, 1.0eq), 3.4g compound 13 (25.0mM, 1.1eq, compound), 6.5g triphenylphosphine (25.0mM, 1.1eq) in 100ml tetrahydrofuran, ice bath Slowly add DEAD3.9ml (25.0mM) dropwise under stirring, react at room temperature for 10h, then add DEAD1.3ml (8.3mM), react at room temperature for 10h, then stop the reaction. After filtration, the filtrate was rotated to evaporate, washed with 0.5N NaOH solution, washed with saturated brine, and dried over anhydrous sodium sulfate. The desiccant was filtered off, the solvent was distilled off under reduced pressure, and the residue was separated by silica gel column chromatography, the eluent was petroleum ether: ethyl acetate = 7:1, and the required components were collected to obtain compound 20 as a pale yel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com