Preparation method of bisbenzimidazole group-containing phenanthroline-type derivative and organic light-emitting device (OLED)
A technology of dibenzimidazolyl phenanthrene and benzimidazolyl phenanthrene, which is applied in the field of organic optoelectronic materials, can solve problems such as no synthesis method, and achieve the effects of good solubility, excellent thermal stability and high glass transition temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example
[0044]
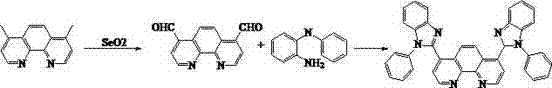
[0045] Add 20.8g (0.1mol) 4,7-dimethyl-1,10-phenanthroline and 49.9g (0.45mol) selenium dioxide to a 5L three-necked flask, add to the mixture of 1300ml dioxane and 55ml water Solution, temperature raised to 100°C, reflux reaction for 3-4 hours, use TLC method to determine the end of the reaction, stop the reaction, pass it through the diatomaceous earth funnel while it is hot, and solid precipitates after cooling, tetrahydrofuran recrystallization to obtain dialdehyde compounds 19.1 g (0.081 mol) of yellow crystals, yield 81%.
[0046] In a 1L three-necked flask, put 19g (0.08mol) of the above dialdehyde compound, 36.6g (0.2mol) of o-aminodiphenylamine, and 38g (0.2mol) of sodium metabisulfite in 400ml of DMF, and reflux for overnight reaction. After the reaction was detected by TLC, cool to room temperature, washed with water, extracted with ethyl acetate, and recrystallized to obtain 33.9 g (0.06 mol) of a white solid with a yield of 75%.
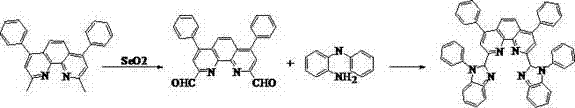
[0047] Synthesis exa...
Embodiment 1
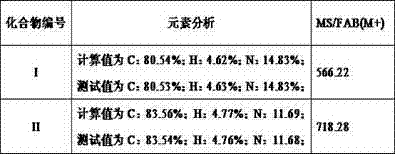
[0064] Measuring Example 1: Luminescent Properties of Compounds of Formula (I), Formula (II) and Comparative Samples
[0065] Under the same conditions, the samples of Comparative Example 1 and the samples of the application examples formula (I) and formula (II) were measured. The measurement uses the KEITHLEY Keithley 235 source measurement unit, Spectrascan PR650 Spectrum scanning colorimeter to evaluate driving voltage, luminous brightness, luminous efficiency, and luminous color. The results are listed in Table 2:
[0066] Table 2
[0067]
[0068] According to Table 2, the above-mentioned samples exhibit blue emission color in the wavelength range of 460-480nm. Compared with the sample of Comparative Example 1 using the sample of Example 1, the organic light emitting device using the organic layer of the dibenzoimidazolylphenanthroline derivative may have lower driving voltage, higher luminance, and higher efficiency.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com