Preparation method of 5,4'-dihydroxy flavone-7-O-D-glucuronic acid
A technology of glucuronic acid and dihydroxyflavone is applied in the field of preparation of 5,4'-dihydroxyflavone-7-O-D-glucuronic acid, which can solve the problems of long synthesis route, difficult extraction, high price, etc. The effect of few steps, simple and easy operation, and cheap and easy-to-obtain starting materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] The preparation of structure compound shown in embodiment 1 formula I
[0082] 1) Preparation of structural compounds shown in formula III
[0083] The reaction equation is as follows:
[0084]
[0085] Wherein, in the compound of formula III, R is a methyl group.
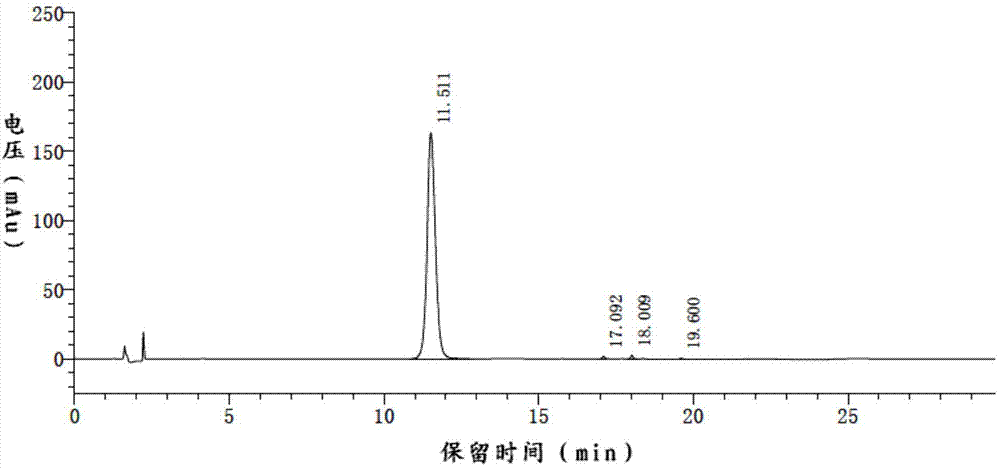
[0086] Add 5.4g (20mmol) of apigenin, 22mL of acetic anhydride (d=1.08g / ml, 233mmol) and 27mL of pyridine in a dry and clean 150mL three-necked round-bottom flask equipped with a condenser, and heat to about 140°C to make The reaction was carried out under reflux for about 8 hours, and the raw materials were detected by TLC to have completely reacted. Stop heating, and when the temperature drops to about 50°C to 70°C, add 60 mL of ethyl acetate, stir and crystallize, filter with suction, wash the filter cake with ethyl acetate, and obtain a white flocculent solid 5,7,4'-tri The acetoxyflavone, namely the compound of formula III, was dried to obtain 6.7 g, with a yield of 90.8%.
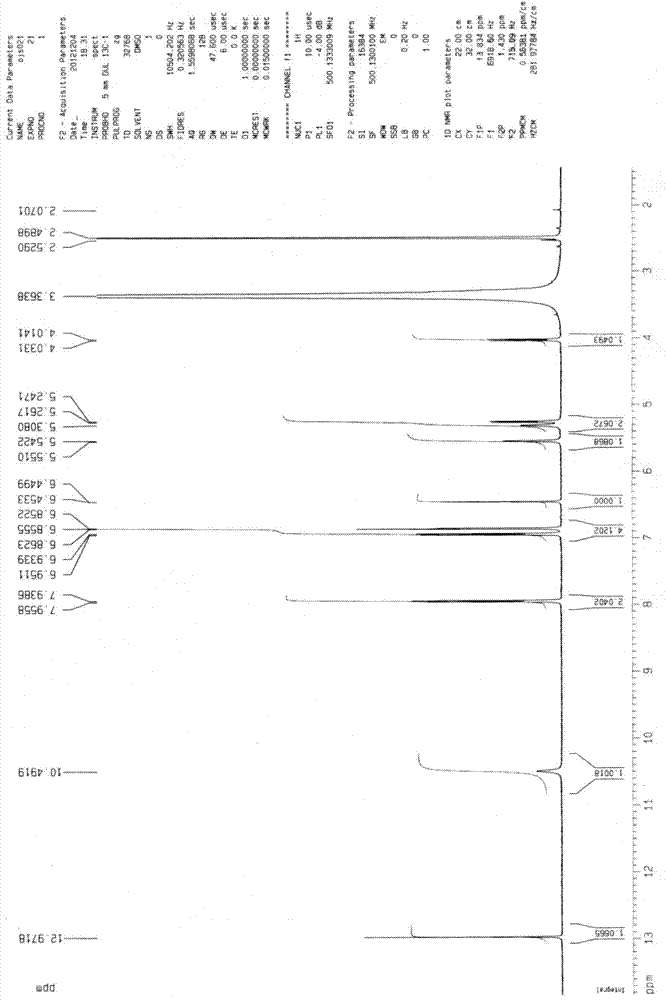
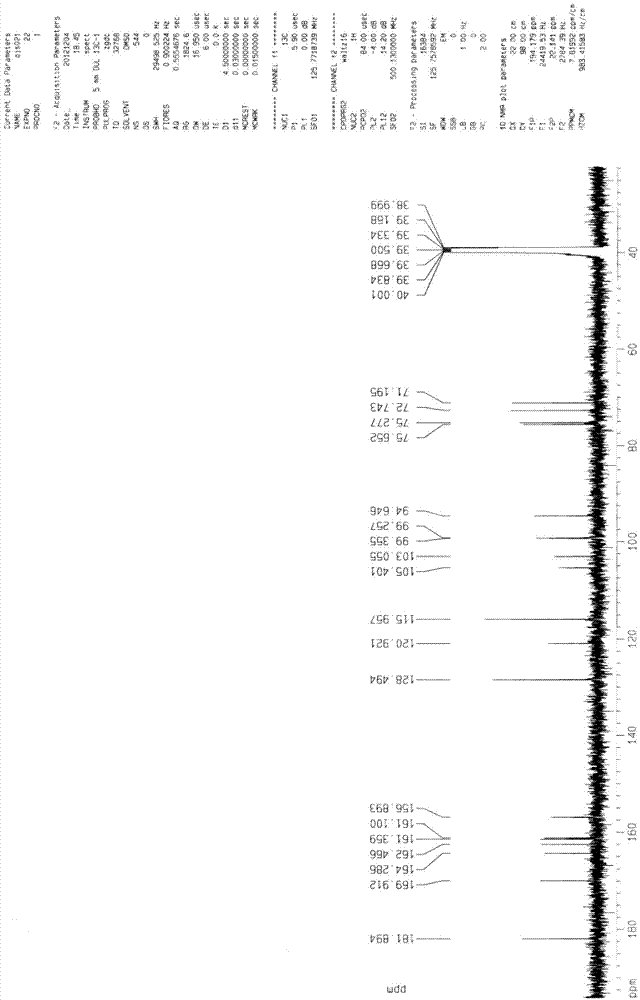
[0087] 1 HNMR(500MHz...
Embodiment 2
[0100]The preparation of structure compound shown in embodiment 2 formula I
[0101] 1) Preparation of structural compounds shown in formula III
[0102] The reaction equation is as follows:
[0103]
[0104] Wherein, in the compound of formula III, R is phenyl.
[0105] Add 80g of pyridine hydrochloride to a 500ml round-bottomed flask, heat to about 140°C to dissolve, add 10g (37mmol) apigenin, and slowly add 70mL of benzoyl chloride (d=1.212, 600mmol) while stirring, and heat to 200°C The reaction was refluxed for about 9 hours. TLC detected that the reaction was complete. When the reaction solution was cooled to about 70°C, 90 mL of ethyl acetate was added, and a large amount of white solids gradually precipitated. Stirring was continued for 1 hour, cooled to room temperature, and left overnight in the refrigerator. Filtration afforded 5,7,4'-tribenzoyloxyflavone as an off-white solid. After drying, recrystallize with ethanol to obtain 19.2g of pure 5,7,4'-tribenzoyl...
Embodiment 3
[0118] The preparation of structure compound shown in embodiment 3 formula I
[0119] 1) Preparation of structural compounds shown in formula III
[0120] The reaction equation is as follows:
[0121]
[0122] Wherein, in the compound of formula III, R is a methyl group.
[0123] Dissolve 5.4g (20mmol) of apigenin in 30ml of pyridine, slowly add 25mL of acetic acid (d=1.049g / ml, 437mmol) under stirring at room temperature, heat to 180°C and reflux for about 8 hours, TLC detects that the reaction is complete, and the reaction solution When it was cooled to about 70°C, 90 mL of ethyl acetate was added, and a large amount of white solid gradually precipitated. Stirring was continued for 1 hour, cooled to room temperature, and left overnight in the refrigerator. Filtration afforded 5,7,4'-triacetoxyflavone as an off-white solid. After drying, it was recrystallized with ethanol to obtain 6.4 g of pure 5,7,4'-triacetoxyflavone (ie, the structural compound shown in formula III...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com