Trastuzumab drug-resistant human breast cancer cell line and preparation method and application thereof
A technology of human breast cancer cells and trastuzumab, which is applied in the field of biomedicine, can solve the problems of inconspicuous drug resistance, and achieve the effects of small growth and proliferation speed, small decrease, and stable cell shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Construction of trastuzumab-resistant human breast cancer cell line BT-474 / HR
[0030] (1) resuscitate common human breast cancer cells BT-474 into T25 culture flasks, add RPMI 1640 medium containing 10% special-grade fetal bovine serum to cultivate, and the T25 culture flasks are placed in a 37°C, 5% CO2 incubator In the process, take the cells in the logarithmic growth phase, add trastuzumab (purchased from Roche, specification 320mg / bottle) to an effective concentration of 10ug / ml, and then replace the same concentration of trastuzumab every 3-4 days When the cells grow to 80% confluence, they are digested with 0.25% trypsin and subcultured into new T25 medium for culture, and the treatment is continued for 2 months;
[0031] (2) Gradually increase the effective concentration of trastuzumab in the culture medium for 3 times: increase the effective concentration of trastuzumab to 20ug / ml, and culture continuously for 2 months; increase the effective concent...
Embodiment 2
[0034] Embodiment 2: Morphological observation of cell lines
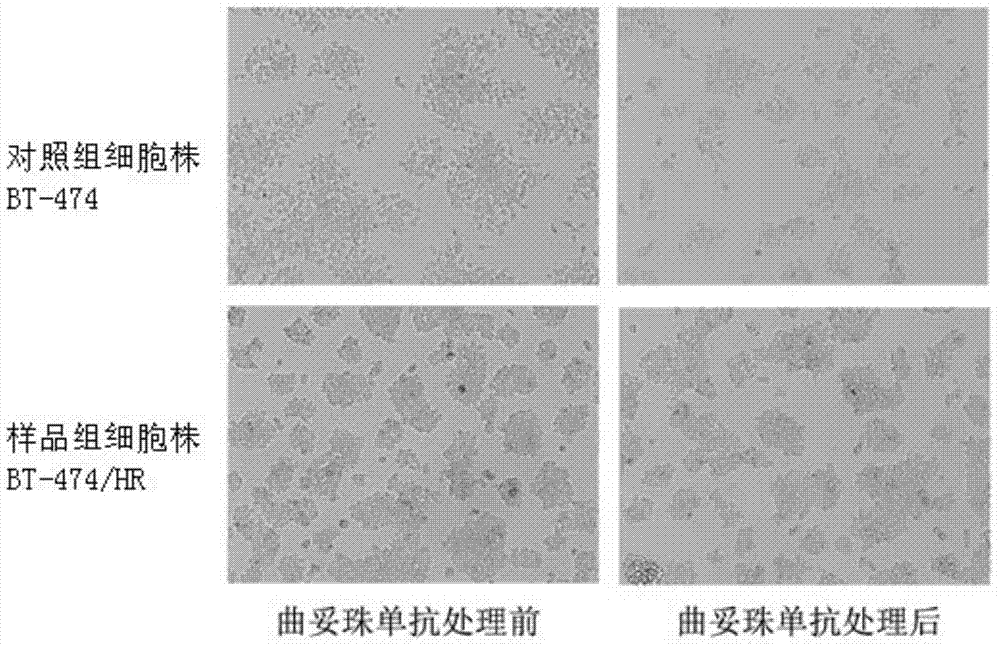
[0035] The trastuzumab-resistant human breast cancer cells BT-474 / HR in the sample group and the common human breast cancer cells BT-474 in the control group were digested with 0.25% trypsin, and then seeded in 6-well cell culture plates, each well containing For 5×105 cells, add RPMI 1640 medium containing 10% special grade fetal bovine serum, culture in an incubator at 37°C and 5% CO2 for 24 hours, take the cells in the logarithmic growth phase, and replace them with 10ug / ml trastuzumab The medium of monoclonal antibody was cultured in an incubator at 37°C and 5% CO2 for 72 hours, and photographed under an inverted phase-contrast microscope to observe the morphology of living cells. The results were as follows: figure 1 As shown, in the absence of trastuzumab, trastuzumab-resistant human breast cancer cells BT-474 / HR (hereinafter referred to as cells BT-474 / HR) and common human breast cancer cells BT- The morpho...
Embodiment 3
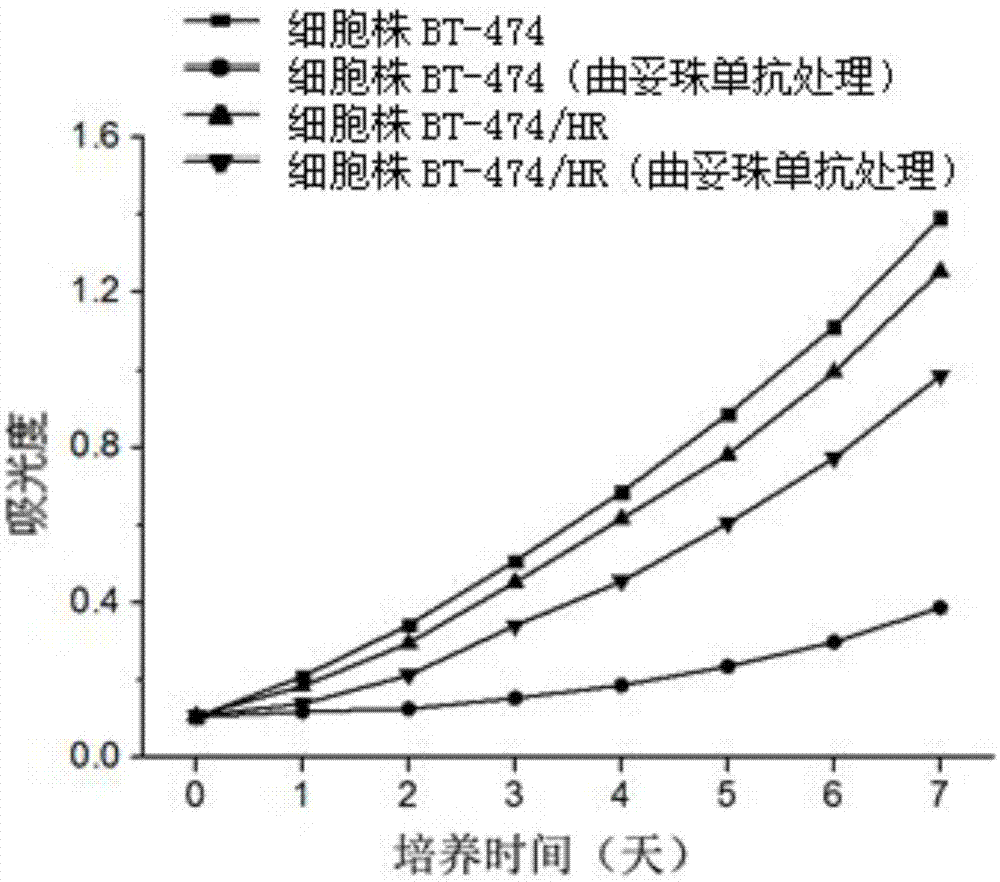
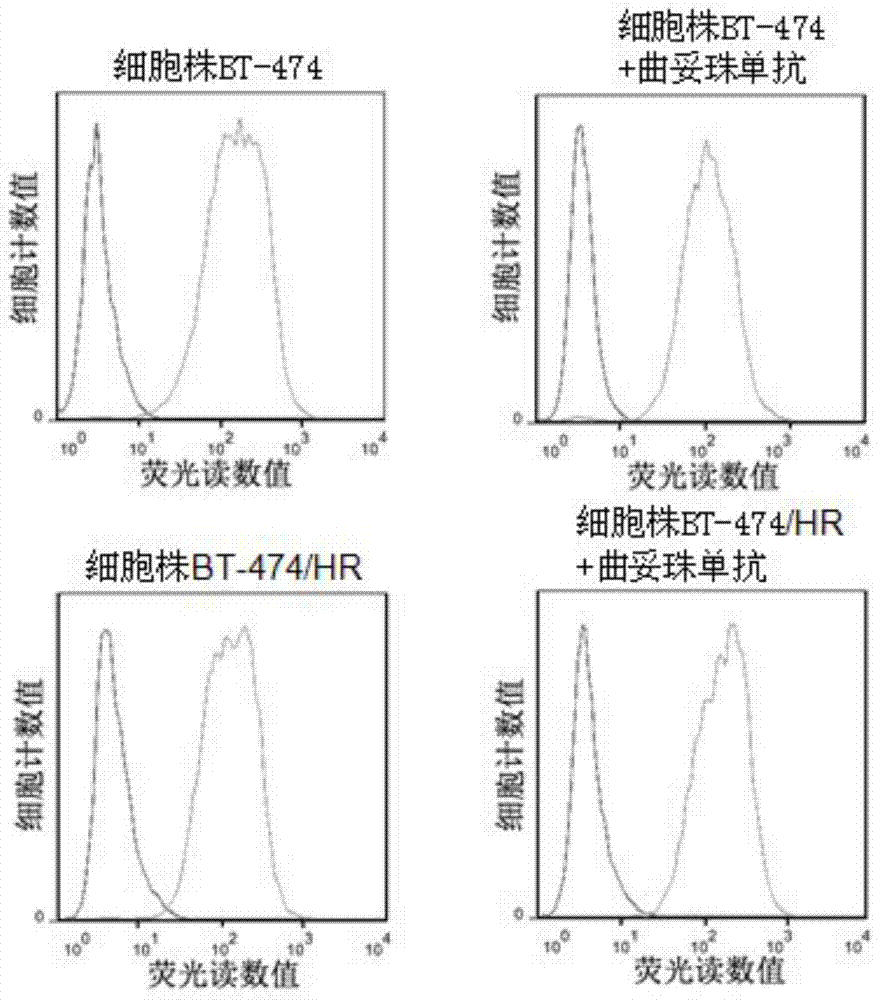
[0036] Example 3: In vitro proliferation of cell lines and their sensitivity to trastuzumab
[0037] Cells BT-474 / HR in the sample group and BT-474 cells in the control group were digested with 0.25% trypsin, and then inoculated in seven 96-well cell culture plates, among which No. 1-7 culture plates were used for culturing plus trastuzumab The above two kinds of cells on the 1st, 2nd, 3rd, 4th, 5th, 6th and 7th day of monoclonal antibody treatment, each cell was inoculated into 12 wells, each well contained 10,000 cells, and 100 μL containing 10% special grade fetal bovine serum was added The RPMI 1640 culture medium was placed in an incubator at 37°C and 5% CO2 for 24 hours, and then replaced with fresh medium (the fresh medium of 6 culture plates inoculated with cells BT-474 / HR and BT-474 was added with 10ug / ml of trastuzumab), and the time at this time was recorded as 0, placed at 37 ° C, 5% CO2 After cultivating in an incubator for 24 hours, according to the CCK-8 kit (pu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com