Technology for biotransformation preparation of panoxadiol saponins by recombinant glucoside hydrolase
A technology of ginsenoside and ginseng diol, which is applied in the direction of biochemical equipment and methods, hydrolytic enzymes, enzymes, etc., and can solve problems such as degradation, inapplicability to industrial-scale applications, and easy mutation of microorganisms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
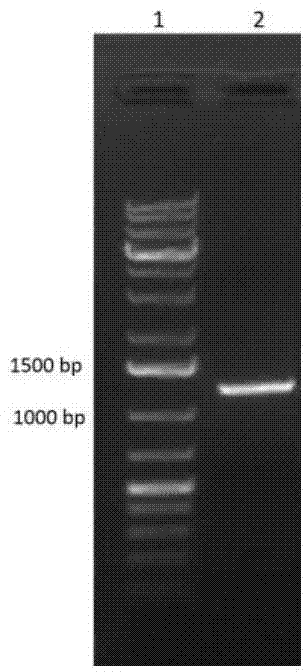
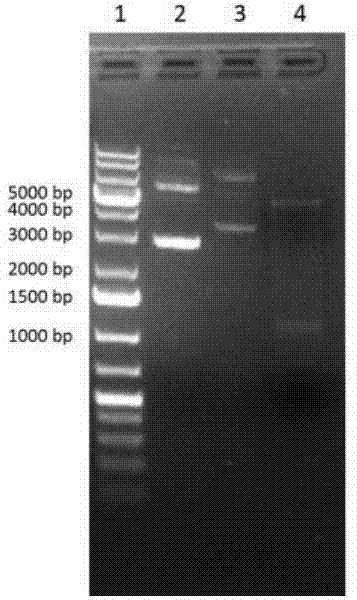
[0076] Embodiment 1, the construction of ginsenoside hydrolase recombinant vector
[0077] The polynucleotide (SEQ ID No.1) of ginsenoside hydrolase from Cellulosimerbium sp. 21 (preservation number: CGMCC No.7587) was obtained by PCR, and cloned into the expression vector pET-28a, A recombinant plasmid capable of expressing ginsenoside hydrolase was obtained. Specific steps include:
[0078] 1) Extraction of Genomic DNA of Cellulosimerbium sp. 21:
[0079] Cellulosimicrobium sp. 21 was activated on LB agar medium at 37° C. for 12 hours. The activated strains were put into LB liquid medium in a ring, and cultured with shaking at 37°C and 200rpm for 12h. Bacterial cells were collected by centrifugation at 5000 rpm and 4°C for 10 min, and Genomic DNA of Cellulosimerbium sp.
[0080] 2) Amplification of CcBgl1A gene:
[0081]The CcBgl1A gene was amplified by PCR using the genomic DNA of Cellulosimicrobium sp. 21 as a template. The amplification primers were: Primer-F 5′-GGA...
Embodiment 2
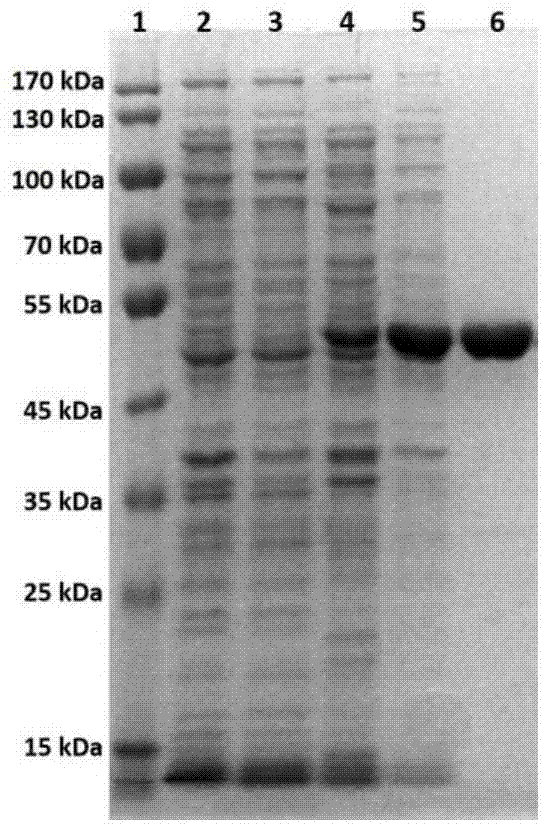
[0085] Embodiment 2, the preparation of recombinant ginsenoside hydrolase CcBgl1A
[0086] 1. The recombinant strain pET-28a / CcBgl1A / E.coli BL21(DE3) of Example 1 was inoculated on LB solid medium containing 50 μg / ml kanamycin, and cultured overnight at 37°C. Pick one ring and inoculate it into 5ml LB liquid medium containing 50μg / ml kanamycin, and culture it at 37°C and 200rpm for 12h (the LB liquid medium containing 50μg / ml kanamycin is used as blank control), which is the seed liquid. The seed solution was inoculated into 200ml LB liquid medium containing 50μg / ml kanamycin at a ratio of 1:50, and cultured in a 1L shake flask at 37°C and 200rpm. When OD600 reached 0.5 (fermentation medium was used as blank control), the temperature was lowered to 25°C, and 0.4mM IPTG was added for induction for 20h. After the induction, collect the bacteria by centrifugation at 5000rpm for 10min, wash the bacteria once with PBS, resuspend in 50ml of PBS buffer, break the cells by ultrasoni...
Embodiment 3
[0098] Embodiment 3, the biological characteristic of recombinant β-glucosidase CcBgl1A
[0099] 1. The effect of pH on the activity of recombinant β-glucosidase CcBgl1A
[0100] Appropriately diluted purified recombinant β-glucosidase CcBgl1A was reacted with substrate pNPG in buffers with different pH values at 37°C for 10 min, and the enzyme activity of recombinant β-glucosidase CcBgl1A at different pH conditions was determined. The buffers used are: pH 2.0-6.0, 50mM NaAc-HAc buffer; pH 6.0-8.0, 50mM Na 2 HPO 4 -NaH 2 PO 4Buffer; pH 8.0-11.0, 50 mM Glycine-NaOH buffer. The experimental results showed that the optimal pH value of recombinant β-glucosidase CcBgl1A was 5.5 ( Figure 6 Middle a).
[0101] 2. Effect of pH on the stability of recombinant β-glucosidase CcBgl1A
[0102] Mix 10 μl of suitably diluted purified recombinant β-glucosidase CcBgl1A enzyme solution with 50 μl of 100 mM buffer solution with various pH values (4.0-10.0) (the buffer solutions used ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com