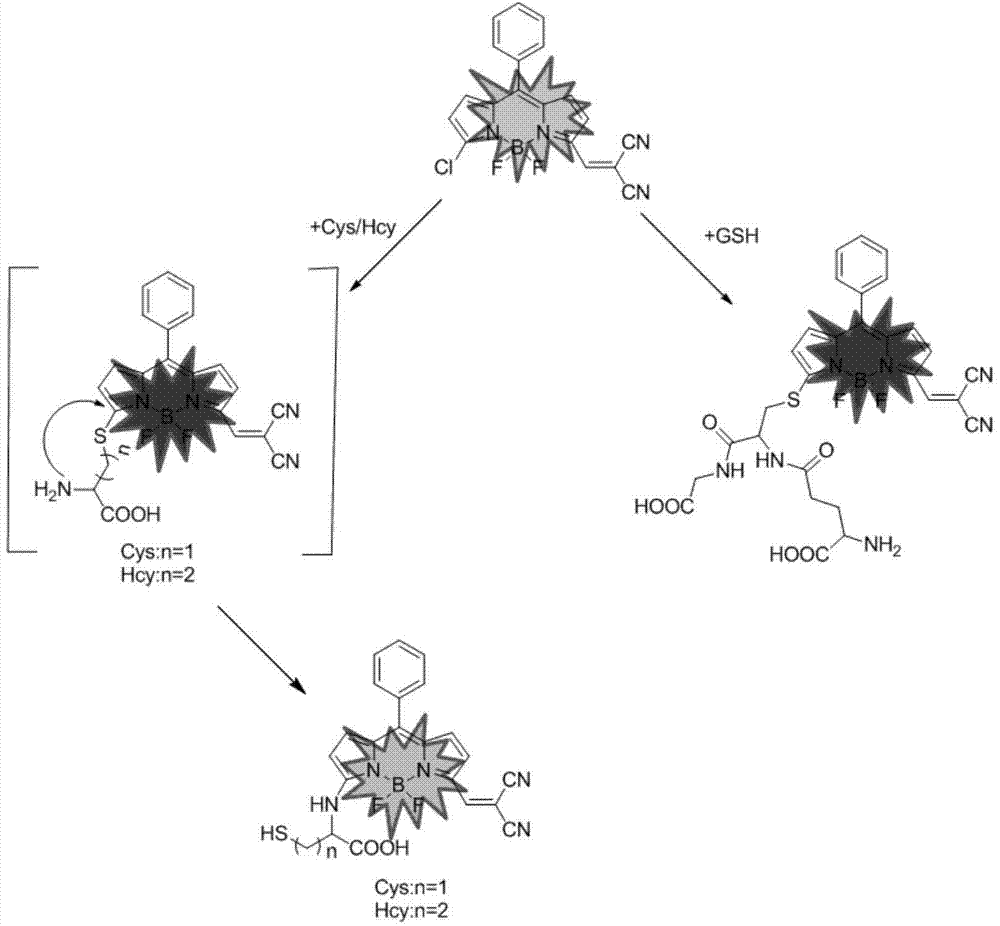
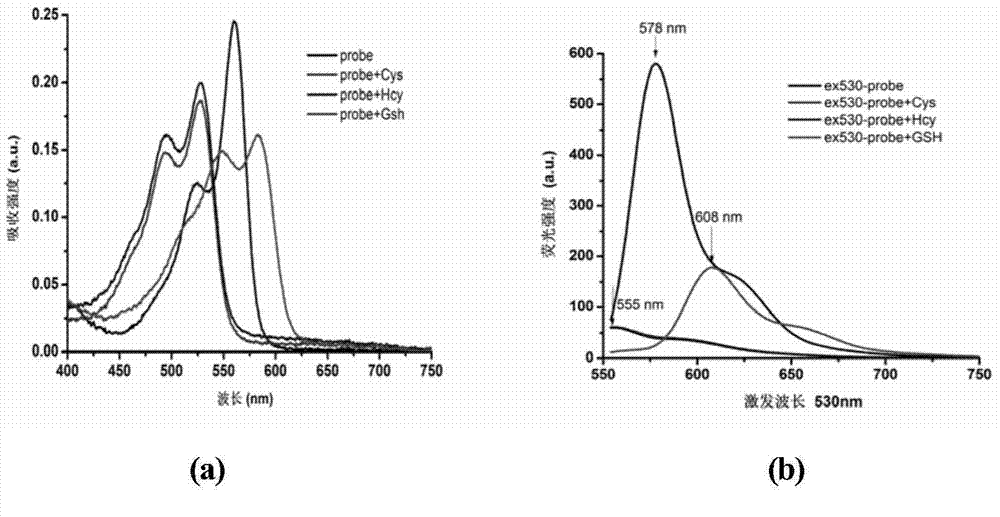
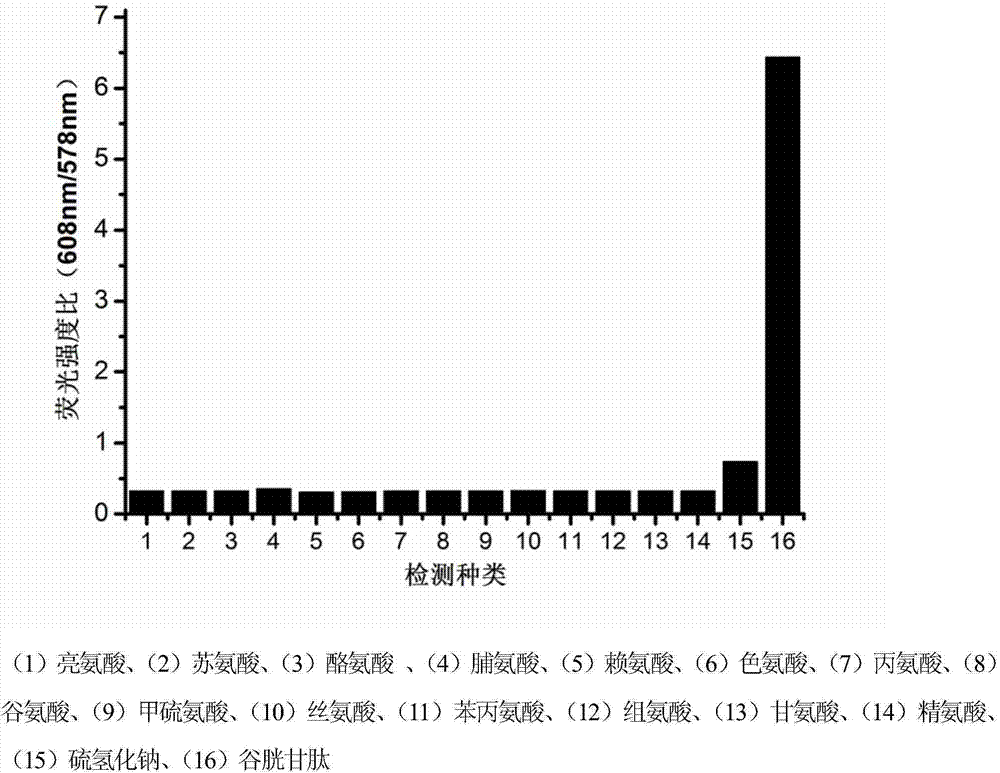
Novel fluorescent dye capable of multifunctionalization, and preparation method and application thereof
A fluorescent dye, multi-functional technology, applied in the field of chemical biology, can solve problems such as cell death and harmful organisms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] Embodiment 1: the synthesis of compound 1
[0097] Benzoylmorpholine (11.64g, 0.061mol) and POCl 3 (7.0ml, 0.077mol) was sequentially added to the round bottom flask, stirred until completely dissolved, and reacted at room temperature until the reaction was complete; then pyrrole (4.2ml, 0.061mol) was slowly added dropwise to the reaction system to react; Completely, put the reaction system into an ice bath, add saturated Na to the reaction system dropwise 2 CO 3 solution until no bubbles were generated; extracted with dichloromethane, dried and then spin-dried to obtain 9.13 g of a white solid. Then the obtained white solid (2.91g, 0.017mol) was dissolved in acetonitrile and weighed CuCl 2 2H 2 O (6.02g, 0.035mol) was added to the reaction system, and after the reaction was complete, slowly drop H into the reaction system 2 SO 4 The solution was stirred evenly, extracted with dichloromethane, dried and spin-dried. Through silica gel column chromatography, 1.65 g...
Embodiment 2
[0098] Embodiment 2: the synthesis of compound 2
[0099] Weigh pyrrolealdehyde (0.95g, 0.01mol) and dissolve it in ethanol, then add propanedicyanide (0.73g, 0.011mol) into the above reaction system, wait for most of the solids to dissolve, then add a small amount of piperidine, The reaction temperature was refluxed at high temperature. After the reaction was complete, the reaction system was lowered to room temperature, and a solid was produced by washing with water to obtain a crude product, which was passed through a silica gel column to obtain 1.08 g of compound 2 as a yellow solid.
Embodiment 3
[0100] Embodiment 3: the synthesis of compound Cl-BODIPY-2CN
[0101] Compound 1 (0.206g, 0.001mol) was weighed and dissolved in dichloromethane, POCl3 (0.136ml, 0.0015mol) was added to the solution, and stirred at room temperature. Then compound 2 (0.172g, 0.0012mol) was dissolved in a small amount of dichloromethane and added to the above reaction system. Under the protection of inert gas, react for 12h. To complete the reaction, add saturated NaHCO 3 Adjust the pH of the solution to neutral, extract, spin dry after drying, dissolve the obtained product in an appropriate amount of dichloromethane, add Et 3 N (0.693ml, 0.005mol) was stirred, then BF was added dropwise 3 ·Et 2 O (0.631ml, 0.005mol), stirred overnight after the dropwise addition, after the reaction was complete, extracted, dried and then spin-dried. After going through a silica gel column chromatography, 0.176 g of the product cyan solid Cl-BODIPY-2CN was obtained; 1 HNMR (CDCl 3 ,400MHz):δ8.29(s,1H),7.6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com