Pichia yeast recombinant bacterial strain highly expressing Neuritin truncated protein, and application thereof
A technology of recombinant strains, Pichia pastoris, applied in the field of microorganisms, can solve the problems of lack of effective treatment and/or preventive drugs for nervous system diseases and nerve injuries, achieve stable passage and large-scale culture, facilitate large-scale culture, and promote regeneration and the effect of functional recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1 Screening of Pichia pastoris recombinant strains highly expressing Neuritin truncated protein
[0032] The screening steps of the Pichia pastoris recombinant strain highly expressing Neuritin truncated protein in the present embodiment are as follows:
[0033] 1. Construction of recombinant Pichia pastoris
[0034] According to the method of Example 1 in Chinese invention patent CN200810227347-"Neuritin truncated protein active fragment and its expression system and special vector", the neuritin truncated body was used as a template to amplify the neuritin truncated body fragment, and the PCR product was used EcoRI, After NotⅠ restriction endonuclease digestion and purification, the digestion products were connected, transfected into DH5α competent cells, and the positive transformants identified as positive by PCR were electrotransformed with Pichia pastoris GS115 competent cells, and the bacterial solution was coated with Spread on the MD plate, cultivate...
Embodiment 2 Embodiment 1
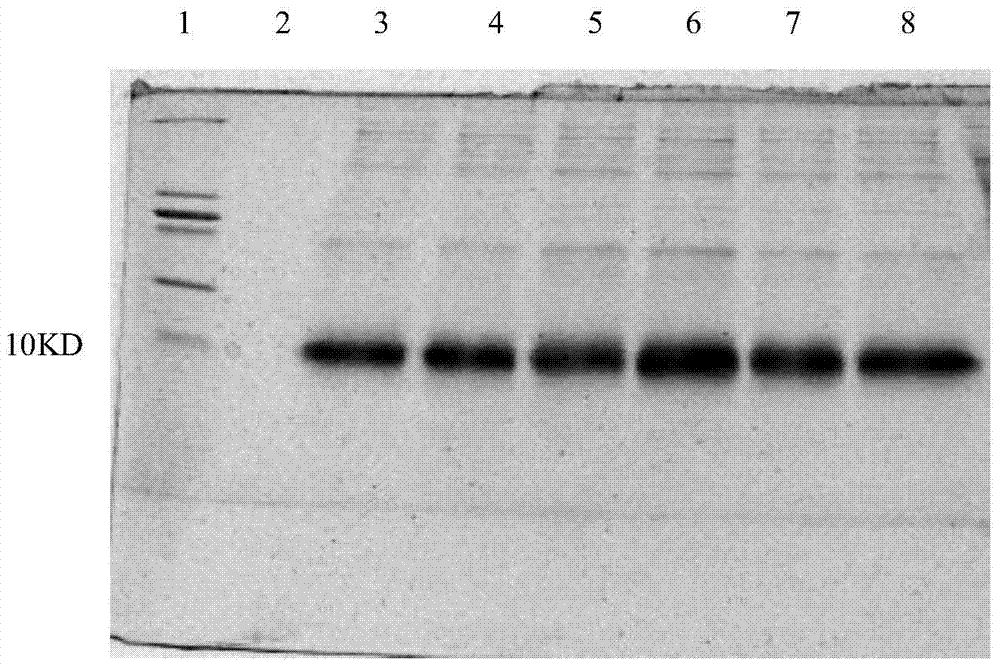
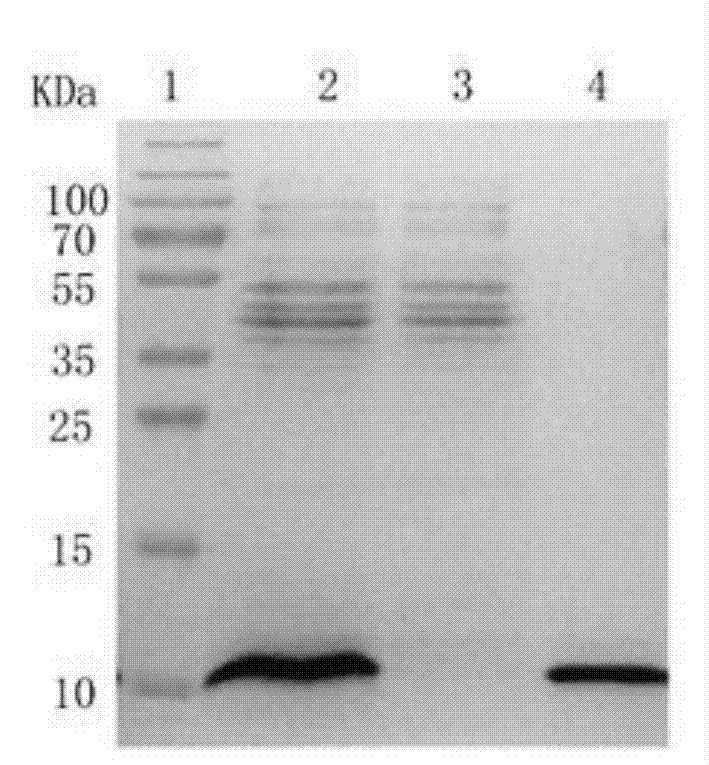
[0052] Example 2 Purification of Pichia recombinant strains highly expressing Neuritin truncated protein obtained through screening in Example 1
[0053] The specific implementation steps are as follows:
[0054] (1) Utilize the highly expressed bacterial strain screened out in Example 1 to induce a large amount of expressed protein;
[0055] (2) After running the AKTA purification system, first wash the affinity chromatography column with 8mol / L urea, 1ml / min, 30min;
[0056] (3) 0.22um filtered ddH 2 O wash affinity chromatography column, 1ml / min, 20min;
[0057] (4) 0.1mol / L NiSO 4 , 1ml / min, 10min;
[0058] (5)ddH 2 O, 1ml / min, 10min;
[0059] (6) 1×start buffer equilibrium chromatography column, 1ml / min, 10min;
[0060] (7) Protein liquid collected, 0.7ml / min;
[0061] (8) 1×start buffer (20mM sodium phosphate, 0.5M NaCl, 10mM imidazole, pH7.4) equilibrates the chromatography column again, 1ml / min, until the protein absorption detection line is balanced;
[0062]...
Embodiment 3
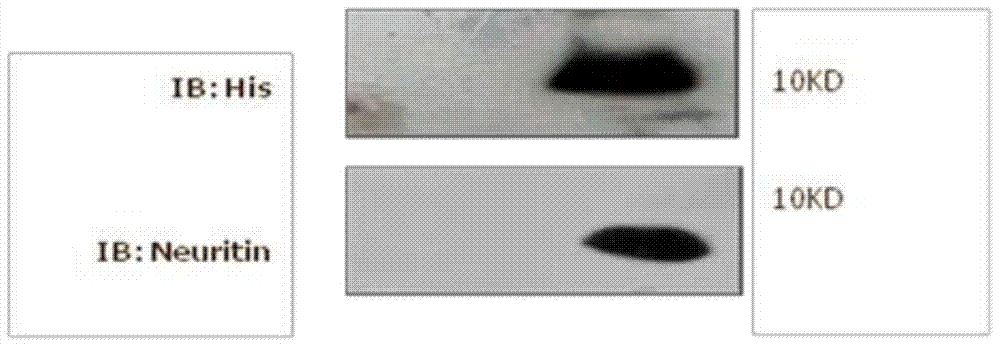
[0068] Example 3 Identification of the Neuritin truncated protein purified in Example 2
[0069] 1. Western blot to identify the expressed protein, the specific implementation steps are as follows:
[0070] (1) Electrophoresis: stacking gel 80v, 30min; separating gel: 110v, 1h.
[0071] (2) Membrane transfer: Use a semi-dry transfer apparatus to transfer the protein from the gel to the PVDF membrane, place it in order from bottom to top: filter paper, membrane, glue, filter paper, and use a test tube to drive out the air bubbles between the layers, set the voltage and time : 21V, 42min.
[0072] (3) Blocking: Place the membrane in 5% skimmed milk powder (TBST dissolved), and block at room temperature for 2 hours on a shaker.
[0073] (4) Incubate the primary antibody: 6×His monoclonal antibody diluted 1:2000 in 5% skimmed milk powder-TBST solution, overnight at 4°C on a shaker.
[0074] (5) Washing: Put the membrane face up, add TBST solution, shake and wash once for 5 minu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com