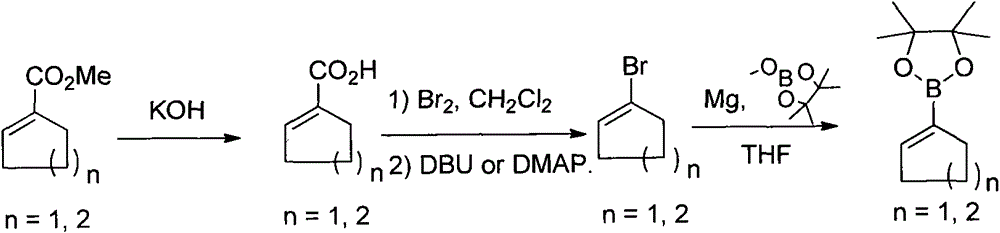
A method of preparing cyclopenten/cyclohexen-1-yl-boronic acid pinacol ester
A technology of cyclopentane, alcohol ester, applied in the field of pharmaceutical intermediate synthesis, can solve the problems of high cost, unsuitable solvent for amplification, etc., and achieves the effects of simple operation, strong continuity and few by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1: a kind of method for preparing cyclopentene-1-boronic acid pinacol ester, comprises the following steps:
[0018] Add 126g (1mol) of methyl cyclopentene-1-carboxylate and 280mL of dichloromethane into the reaction flask, slowly add 500mL (1.5mol) of 3M KOH aqueous solution at room temperature, after the addition is complete, stir and react for 1-2 hours, After TLC detects that the raw materials disappear, add 2M hydrochloric acid to adjust the pH to 4-5, separate the organic layer, add 150mL dichloromethane to the aqueous layer, separate the layers again, combine the organic layers, and wash with saturated brine to obtain cyclopentene The dichloromethane solution of -1-carboxylic acid, with a purity of more than 98% (after deducting the solvent) as detected by GC, was directly used in the next reaction.
[0019] Add the dichloromethane solution of the above-mentioned cyclopentene-1-carboxylic acid in the reaction flask equipped with a dropping funnel, a re...
Embodiment 2
[0021] Embodiment 2: a kind of method for preparing cyclohexene-1-boronic acid pinacol ester, comprises the following steps:
[0022] Add 70g (0.5mol) of methyl cyclohexene-1-carboxylate and 200mL of dichloromethane into the reaction flask, slowly add 250mL (0.75mol) of 3M KOH aqueous solution at room temperature, and stir for 1-2 hours after the addition is complete , after the disappearance of the raw materials detected by TLC, 6M hydrochloric acid was added to adjust the pH to 4-5, the organic layer was separated, and 100 mL of dichloromethane was added to the aqueous layer. The dichloromethane solution of alkene-1-carboxylic acid has a purity of more than 97% (after deducting the solvent) as detected by GC, and is directly used in the next reaction.
[0023] Add the dichloromethane solution of the above-mentioned cyclohexene-1-carboxylic acid in the reaction flask equipped with a dropping funnel, a reflux condenser and a distillation device, and add bromine 76.8g (0.48mol)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com