MUC1-Fc polypeptide vaccine as well as preparation method and application thereof
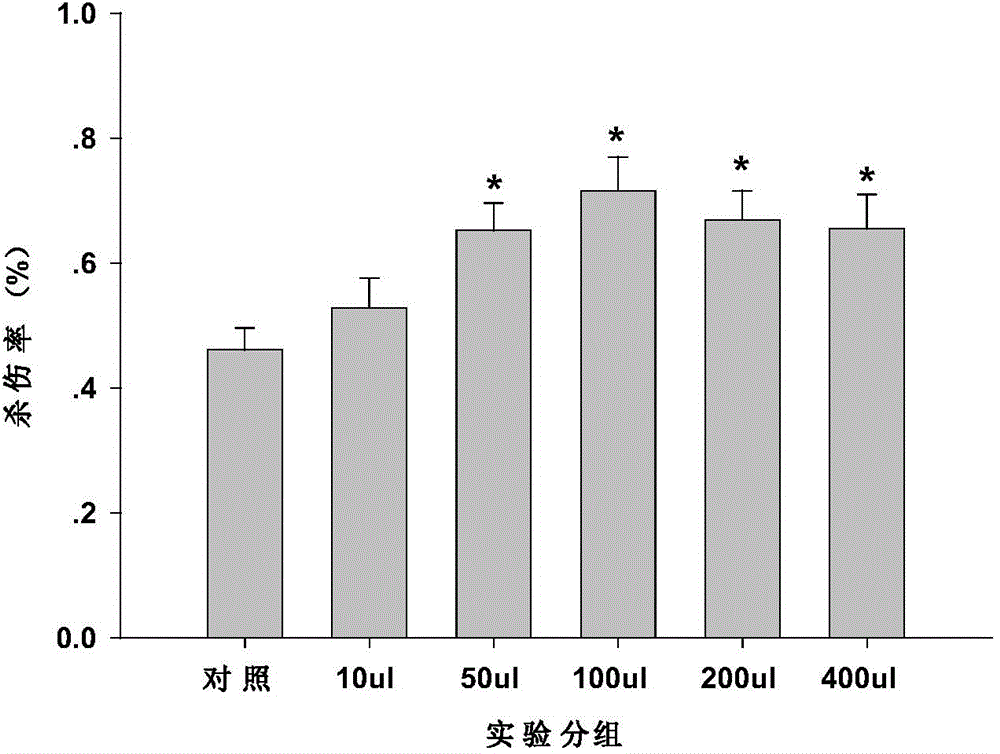
A polypeptide vaccine, muc1-fc technology, applied in the field of tumor DNA vaccines and viral vector vaccines, can solve problems such as ineffective activation of CTL responses, and achieve the goal of improving antigen bioavailability, good preventive and therapeutic effects, and increased killing rate. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
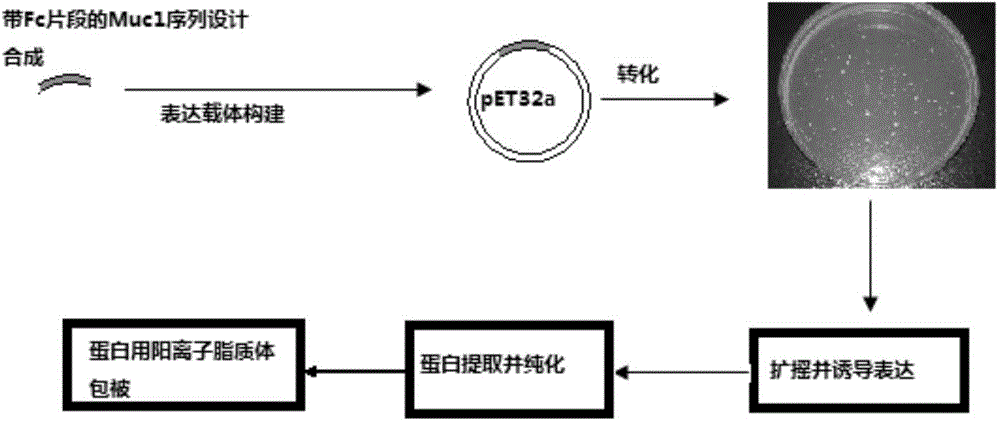
[0046] The preparation method of the MUC1 antigen polypeptide with immunoglobulin Fc segment specifically comprises the following steps:
[0047] Fusing the immunoglobulin Fc segment to the MUC1 polypeptide segment to obtain the MUC1 antigen polypeptide sequence with the immunoglobulin Fc segment, its sequence is shown in SEQ ID NO: 1, using the Red / ET homologous recombination method to design the upstream homology arm: (HindIII) CTCTAGCGTTTAAACTT AAGCTT ; downstream homology arm: (BamHI) GGATCC ACTAGTCCAGTGTGGTGGA, the MUC1 antigen polypeptide sequence with immunoglobulin Fc segment is constructed on the pET32a vector, and then expressed and purified in Escherichia coli to obtain the MUC1 antigen polypeptide with immunoglobulin Fc segment.
Embodiment 2
[0049] The preparation method of the MUC1 antigen polypeptide with immunoglobulin Fc segment specifically comprises the following steps:
[0050] Fuse the immunoglobulin Fc segment to the MUC1 polypeptide fragment to obtain the MUC1 antigen polypeptide sequence with the immunoglobulin Fc segment, the sequence of which is shown in SEQ ID NO: 1, using the enzyme-linking method, the restriction site (HindIII) AAGCTT (BamHI) GGATCC, the MUC1 antigen polypeptide sequence with the Fc segment of the immunoglobulin is constructed on the pET29a vector, and then expressed and purified in E. coli to obtain the MUC1 antigen polypeptide with the Fc segment of the immunoglobulin.
Embodiment 3
[0052] Utilize 1,2-dioleoyl-3-trimethylaminoneptane (DOTAP) to coat the MUC1 antigen polypeptide with the immunoglobulin Fc segment of Example 1 to obtain a polypeptide vaccine, and its preparation method comprises the following steps:
[0053] 1) Solvent preparation: prepare a mixture of chloroform and methanol, wherein the volume ratio of chloroform and methanol is 2:1;
[0054] 2) Dissolving 1,2-dioleoyl-3-trimethylaminopropane (DOTAP): Dissolve DOTAP with the solvent in step 1) in a round bottom bottle until the concentration is 1 μM / mL;
[0055] 3) Dry the solvent with a constant flow of nitrogen, so that DOTAP forms a uniform layer of film structure on the container wall;
[0056] 4) After the solvent is blown dry, put it into a vacuum drying oven to dry overnight, so that the solvent can be removed as much as possible;
[0057] 5) Add the MUC1 antigen polypeptide with the immunoglobulin Fc segment of Example 1 diluted with PBS that needs to be coated. The volume should...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com