Method for preparing nicosulfuron
A technology of nicosulfuron and its compound, which is applied in the field of preparation of nicosulfuron, can solve the problems of environmental hazards, high cost, low yield, etc., and achieve the effects of production economy and environmental protection, fewer reaction steps, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
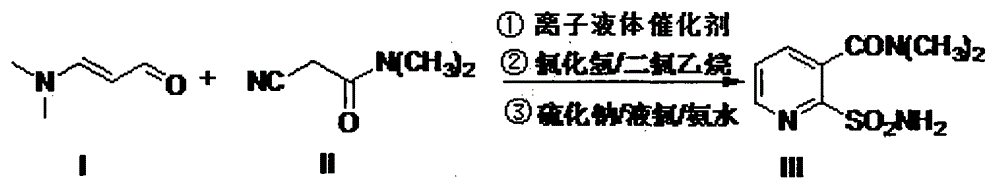
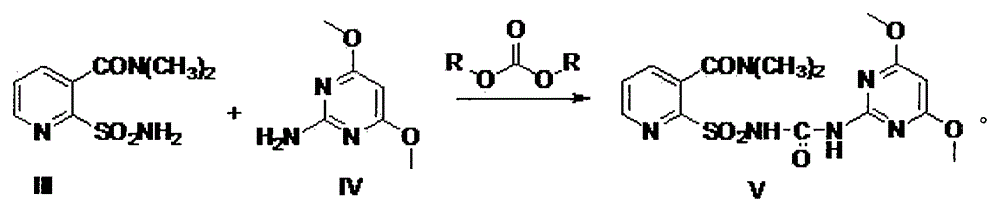
[0022] Step a: Take 1.0mol (99g) of dimethylaminoacrolein (compound I), 1.1mol (123g) of cyanoacetamide (compound II), and 300mL of reaction solvent toluene into the reactor, and then add 0.2mol Pyridine (15.8g), 0.2mol acetic acid (12g), add water separator, then be heated to 120 DEG C and reflux water diversion for 2 hours, reaction solution is cooled to 25 DEG C and pass into excess hydrogen chloride gas in reaction solution, (hydrogen chloride The molar ratio of gas to compound I is 2-5:1, that is, 2:1, 3:1, 4:1, 5:1, or any other ratio within this range), and continue the heat preservation reaction for 2 hours Finally, adjust the pH to weakly alkaline, remove toluene under reduced pressure; add 200mL of water, 2.0mol 70% sodium sulfide (222.8g) and heat to reflux at 120°C for 2 hours, adjust the pH to weakly acidic, add 300mL of 1,2-dichloroethyl Alkanes, stirred for 10 minutes, quickly added 1.5mol liquid chlorine (106.5g) to the mixed phase while stirring at 0-5°C and c...
Embodiment 2
[0027] Step a: Take 1.0mol (99g) dimethylaminoacrolein (compound I), 1.2mol (134g) cyanoacetamide (compound II), and 300mL reaction solvent toluene into the reactor, and then add 0.12mol Piperidine (10.2g), 0.12mol formic acid (5.5g), add a water separator, then heat to 110°C for reflux and water separation for 2 hours, then cool the reaction solution to 0°C and pass excess hydrogen chloride into the reaction solution Gas, (the molar ratio of hydrogen chloride gas to compound I is 2-5:1, that is, 2:1, 3:1, 4:1, 5:1, or any other ratio within this range), continue After 2 hours of heat preservation reaction, adjust the pH to weakly alkaline, and remove toluene under reduced pressure; add 200mL of water, heat 1.5mol 70% sodium sulfide (167g) to 80°C, keep the temperature for 2 hours, adjust the pH to weakly acidic, add 300mL1,2 -dichloroethane, stirred for 10 minutes, quickly added 1.3mol liquid chlorine (92.3g) to the mixed phase while stirring at 0-5°C and continued stirring f...
Embodiment 3
[0030] Step a: Take 1.0mol (99g) dimethylaminoacrolein (compound I), 1.5mol (168g) cyanoacetamide (compound II), and 300mL reaction solvent toluene into the reactor, and then add 0.1mol Triethylamine (10.1g), 0.1mol oxalic acid (9g), add a water separator, then heat to 120°C for reflux and water separation for 2 hours, then cool the reaction solution to 10°C and feed excess hydrogen chloride gas into the reaction solution , (the mol ratio of hydrogen chloride gas and compound I is 2-5: 1, can be 2: 1, 3: 1, 4: 1, 5: 1, also can be other any ratio in this range), continue to insulate After reacting for 2 hours, adjust the pH to weakly alkaline, remove toluene under reduced pressure; add 200mL of water, 1.2mol 70% sodium sulfide (133.7g) and heat to 110°C for 2 hours, adjust the pH to weakly acidic, add 300mL of 1,2- Dichloroethane, stirred for 10 minutes, quickly added 1.1mol liquid chlorine (78g) to the mixed phase while stirring at 0-5°C and continued to stir for 30 minutes, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com